If we do believe it, then we should start drinking EVOO. (I like EVOO, but 4/5 of a cup doesn't seem very appealing.)
You can do it for a week or two, right?

Posted 18 June 2012 - 09:21 PM
If we do believe it, then we should start drinking EVOO. (I like EVOO, but 4/5 of a cup doesn't seem very appealing.)
Posted 18 June 2012 - 09:36 PM
Posted 18 June 2012 - 09:51 PM
Aggregates are filtered out, so if the hypothesis were that the tyrosol is sneaking in by hiding inside a very small aggregate that managed to pass through the filter, I don't think there could be enough mass of C60 in that form to bring in enough tyrosol to matter.
Posted 18 June 2012 - 10:00 PM
What's the theory behind a week or two of 4/5 cup of EVOO producing a marked effect on longevity versus smaller amounts over a longer period of time?
CONCLUSION:
This study shows that intake of virgin olive oil based breakfast, which is rich in phenol compounds is able to repress in vivo expression of several pro-inflammatory genes, thereby switching activity of peripheral blood mononuclear cells to a less deleterious inflammatory profile. These results provide at least a partial molecular basis for reduced risk of cardiovascular disease observed in Mediterranean countries, where virgin olive oil represents a main source of dietary fat.
Edited by Turnbuckle, 18 June 2012 - 10:31 PM.
Posted 18 June 2012 - 10:07 PM
Posted 19 June 2012 - 12:14 AM
Posted 19 June 2012 - 01:25 AM
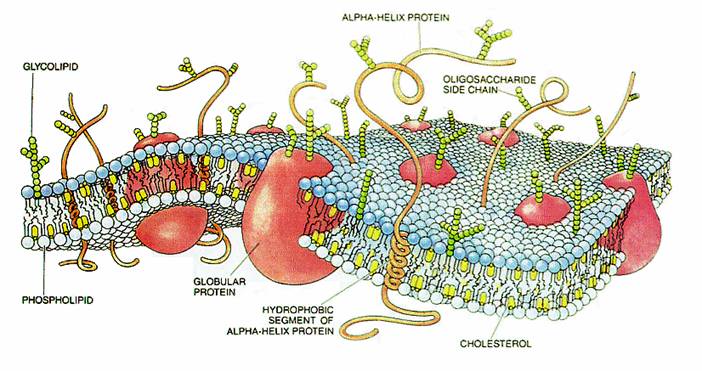

Experimental confirmation (2011) that fullerene is taken up and incorporated in cell membranes:
http://pubs.rsc.org/...1/CC/c1cc14650e
Posted 19 June 2012 - 01:38 AM

Edited by Metrodorus, 19 June 2012 - 01:56 AM.
Posted 19 June 2012 - 01:53 AM
...snip...
That said, I believe the "Cell-Membrane-ROS-Scavenging-As-Essential Mechanism-Explanation" folks should have have a backup hypothesis. The longer term life span enhancing effects experienced by the Baati rats have to be explained somehow. Earlier up thread, niner had tied these effects specifically to the C60 extended stay in the membrane hypothesis.
...snip...
Posted 19 June 2012 - 01:55 AM
In that image, taken from the article http://pubs.rsc.org/...1/CC/c1cc14650e linked by Metrodorus, we can see that the C60 is pushing the phospholipids up out of the membrane plane, which is likely to cause reduced mobility and other issues. If the benefit provided by the C60 outweighs these issues, that's fine.
Edited by niner, 19 June 2012 - 02:00 AM.
Posted 19 June 2012 - 02:01 AM
Posted 19 June 2012 - 03:25 AM
...snip...
That said, I believe the "Cell-Membrane-ROS-Scavenging-As-Essential Mechanism-Explanation" folks should have have a backup hypothesis. The longer term life span enhancing effects experienced by the Baati rats have to be explained somehow. Earlier up thread, niner had tied these effects specifically to the C60 extended stay in the membrane hypothesis.
...snip...
I have to disagree; it is enough.
Pigeons have a 5-fold higher maximum lifespan than rats (35 vs. 7 years) with a similar weight and metabolic rate. Birds have vastly superior lungs and mitochondria compared to mammals, and live far longer.
Improved mitochondria function alone is enough.
Posted 19 June 2012 - 03:25 AM
If the fullerene adducts generated in olive oil accumulate in the liver, then they may be redistributed from there to other organs.
Microscopic examination at D8 of the spleen reticuloendothelial system (RES), where the highest concentrations are observed, shows the presence of some C60 aggregates that are larger and more numerous after i.p. administration (Fig. 2c and d) than after o.g. (Fig. 2a, b): thus C60 concentrations reached the limit of solubility in spleens. In contrast there are no observable deposits inside the livers in all cases indicating that C60 concentrations in these organs are not high enough to trigger precipitation.
While transmission electron microscopy (TEM) at D8 after i.p. administration shows numerous spleen macrophages laden C60 crystals (Fig. 2e) only some C60 crystals were observed inside liver macrophages and very rare crystals in lung (Fig. 2f) and kidney cells (Fig. 2g).
Macrophages Are the Major Source of Spleen TNF During Endotoxemia... We previously established that the cholinergic antiinflammatory pathway regulates TNF production in the spleen (12), but the cellular source of spleen TNF during endotoxemia was previously unknown. Macrophages, a component of the reticuloendothelial system, are a major source of TNF in endotoxemia (18).
Going back to that TNF-alpha discussion earlier - there is one fullerene study that explicitly included TNF-alpha in the experimental design:
http://www.ncbi.nlm....les/PMC2775692/
The key finding was:
C60 significantly suppressed the TNF-α-induced production of proinflammatory cytokines in synovial fibroblasts, synovial infiltrating lymphocytes and macrophagesin vitro.
Edited by wccaguy, 19 June 2012 - 04:00 AM.
Posted 19 June 2012 - 04:24 AM
Edited by wccaguy, 19 June 2012 - 04:25 AM.
Posted 19 June 2012 - 04:32 AM
I agree that improving mitochondrial function is huge, but I think what wccaguy wants to know is, what's the backup explanation if it should be demonstrated that the C60-fatty acid adducts don't hang around for very long? I can't think of a good backup that doesn't involve a lot of hand waving, but then again, do we need a backup?
Edited by wccaguy, 19 June 2012 - 04:34 AM.
Posted 19 June 2012 - 04:49 AM
Posted 19 June 2012 - 04:51 AM
Meanwhile, Kevin Tracey et al, in a 2008 study of the spleen, TNF, macrophages, and their role in the Cholinergic Anti-Inflammatory Pathway found...
Macrophages Are the Major Source of Spleen TNF During Endotoxemia... We previously established that the cholinergic antiinflammatory pathway regulates TNF production in the spleen (12), but the cellular source of spleen TNF during endotoxemia was previously unknown. Macrophages, a component of the reticuloendothelial system, are a major source of TNF in endotoxemia (18).
Given the profound relationships TNF has with several different dimensions of Longevity, including with major Longevity Genes and in Human Population Studies, this seems important.
Posted 19 June 2012 - 07:13 AM
Edited by Metrodorus, 19 June 2012 - 07:19 AM.
Posted 19 June 2012 - 11:32 AM
Edited by wccaguy, 19 June 2012 - 11:33 AM.
Posted 19 June 2012 - 11:55 AM
Edited by wccaguy, 19 June 2012 - 12:06 PM.
Posted 19 June 2012 - 12:05 PM
In the last few days, in multiple posts, the idea has been floating around that C60 accumulates entirely/mostly/importantly in the liver. I understand the idea came from some more general study. That's not what the Baati study shows.
The Baati study says that the organs showing evidence of C60 accumulation were the blood, liver, urine, spleen, and brain. More specifically, the Baati rats showed "numerous spleen macrophages laden C60 crystals."
Microscopic examination at D8 of the spleen reticuloendothelial system (RES), where the highest concentrations are observed, shows the presence of some C60 aggregates that are larger and more numerous after i.p. administration (Fig. 2c and d) than after o.g. (Fig. 2a, b): thus C60 concentrations reached the limit of solubility in spleens. In contrast there are no observable deposits inside the livers in all cases indicating that C60 concentrations in these organs are not high enough to trigger precipitation.
While transmission electron microscopy (TEM) at D8 after i.p. administration shows numerous spleen macrophages laden C60 crystals (Fig. 2e) only some C60 crystals were observed inside liver macrophages and very rare crystals in lung (Fig. 2f) and kidney cells (Fig. 2g).
The rat spleen C60s, "laden with macrophages" were important enough to the Baati team that they thought to include snapshots of these C60s as the Figure 2 series.
Meanwhile, Kevin Tracey et al, in a 2008 study of the spleen, TNF, macrophages, and their role in the Cholinergic Anti-Inflammatory Pathway found...
Macrophages Are the Major Source of Spleen TNF During Endotoxemia... We previously established that the cholinergic antiinflammatory pathway regulates TNF production in the spleen (12), but the cellular source of spleen TNF during endotoxemia was previously unknown. Macrophages, a component of the reticuloendothelial system, are a major source of TNF in endotoxemia (18).
Given the profound relationships TNF has with several different dimensions of Longevity, including with major Longevity Genes and in Human Population Studies, this seems important.
Posted 19 June 2012 - 12:07 PM
Edited by Turnbuckle, 19 June 2012 - 01:06 PM.
Posted 19 June 2012 - 12:16 PM
Meanwhile, Kevin Tracey et al, in a 2008 study of the spleen, TNF, macrophages, and their role in the Cholinergic Anti-Inflammatory Pathway found...
Macrophages Are the Major Source of Spleen TNF During Endotoxemia... We previously established that the cholinergic antiinflammatory pathway regulates TNF production in the spleen (12), but the cellular source of spleen TNF during endotoxemia was previously unknown. Macrophages, a component of the reticuloendothelial system, are a major source of TNF in endotoxemia (18).
Given the profound relationships TNF has with several different dimensions of Longevity, including with major Longevity Genes and in Human Population Studies, this seems important.
Here's the key Kevin Tracey led study, published before the one above, that demonstrated the spleen was a significant source of TNF in the blood...
Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis
Posted 19 June 2012 - 12:20 PM
I think you're taking a wrong turn here. Macrophages do all kinds of things. They can be involved in inflammatory processes typically aimed at attacking microbes, in which they produce TNF. One of their other jobs is eating junk, like said microbes, or any particles that find their way into general circulation, which would include C60 aggregates. These are separate effects; phagocytosing a C60 particle after an ip megadose wouldn't be likely to induce or repress TNF production in general.
Note that this C60 aggregation was a consequence of i.p. injection, which is a good reason to take this stuff orally rather than jabbing a needle in your tummy. It was also a consequence of a 4mg/kg dose, IIRC, which would also be ill-advised.
Posted 19 June 2012 - 12:24 PM
I changed the emphasis in the above quote to point out that this example of splenic involvement is specific to lethal endotoxemia/sepsis. That's a radically different situation than when the spleen picks up particulate junk in a healthy but very heavily dosed animal. I don't think that we can connect the two just because the spleen is involved in both cases.
Edited by wccaguy, 19 June 2012 - 12:24 PM.
Posted 19 June 2012 - 05:25 PM
Posted 19 June 2012 - 07:59 PM
The C60s ate the macrophages in the Baati rats and not the other way around.
Posted 19 June 2012 - 08:07 PM
Mathematical modelling of fullerenes of various diameters in lipid bilayers (full article) (2011)
http://utd.edu/~son0...entnano2011.pdf
Posted 19 June 2012 - 08:14 PM
The C60s ate the macrophages in the Baati rats and not the other way around.
That can't be right. Macrophages are about a thousand times larger than a C60 molecule, for one thing. I thought that the C60 came out of solution in the form of a crystal or aggregate, and these were seen in the macrophages... There's really no way I can see that C60 could eat a macrophage.
Edited by wccaguy, 19 June 2012 - 08:31 PM.
Posted 19 June 2012 - 08:37 PM
I'll look to see what the C60 did... Meanwhile, we do know, from the study Metrodorus posted, that C60 reduces TNF leading to reduction of ROS, damage, and oxidation for cell mitochondria per the studies I posted yesterday.
http://www.ncbi.nlm....pubmed/17999162
Pharm Res. 2008 Jun;25(6):1365-76.
Modulation of tumor necrosis factor-mediated cell death by fullerenes.
Harhaji L, Isakovic A, Vucicevic L, Janjetovic K, Misirkic M, Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S, Nikolic Z, Trajkovic V.
Institute for Biological Research, Belgrade, Serbia.
PurposE: The fullerene (C60/C70 mixture-C60/70) nanocrystalline suspension prepared by solvent exchange method using tetrahydrofyran (THF/nC60/70) and polyhydroxylated C60/70 [C60/70(OH)n] were compared for their ability to modulate cytotoxicity of the proinflammatory cytokine tumor necrosis factor (TNF).
MATERIALS AND METHODS:
TNF-induced cytotoxicity was assessed in L929 fibrosarcoma cells by crystal violet assay. The type of cell death (apoptosis/necrosis), production of reactive oxygen species, mitochondrial depolarization and caspase activation were determined by flow cytometry using the appropriate reporter dyes.
RESULTS:
THF/nC60/70 augmented, while C60/70(OH)n reduced the cytotoxicity of TNF. The numbers of cells undergoing apoptosis/necrosis, as well as of those displaying the activation of apoptosis-inducing enzymes of caspase family, were respectively increased or reduced by THF/nC60/70 or C60/70(OH)n. The antioxidant N-acetylcysteine and mitochondrial permeability transition inhibitor cyclosporin A each partly blocked the cytotoxic action of TNF, indicating the involvement of oxidative stress and mitochondrial dysfunction in the TNF cytotoxicity. Accordingly, THF/nC60/70 or C60/70(OH)n potentiated or suppressed, respectively, TNF-triggered oxidative stress and mitochondrial depolarization.
CONCLUSION:
The ability of different fullerene preparations to modulate TNF-induced oxidative stress and subsequent cell death suggests their potential value in the TNF-based cancer therapy or prevention of TNF-dependent tissue damage.
PMID: 17999162
Edited by niner, 19 June 2012 - 08:39 PM.
0 members, 3 guests, 0 anonymous users