I have had incredible success with inhibiting KYNA metabolites using norharman derived from Syrian Rue seeds (Peganum Harmala), sold as esphand in Iranian/Persian grocery stores. Previously I was using sarcosine but it appears that norharman is much more efficient because it targets the actual problem which sarcosine is meant to target: the inactivation of many pathways by KYNA and its neurotoxic metabolites. Take a good look at the pharmacology below, many receptors are hit - AMPA, NMDA, kainate, the all-important alpha-7 nicotinic acetylcholine receptor.
So rather than looking for an a7 ligand, how about inhibiting KYNA, an the endogenous a7 antagonist?
KYNA does have a very important purpose. It inhibits the convulsive and excitotoxic effects of too much glutamate transmission. However its metabolites like quinolinic acid cause brain fog in the body.
Taking in copious amounts of harmala alkaloids has lead to my brain fog disappearing entirely, replaced by a crystal clarity comparable to Noopept without actually taking Noopept.
It seems somewhere in the pathways, schizophrenics have an L-tryptophan metabolism issue. Instead of getting converted to 5-HT, it might be getting used more for KYNA to neutralise whatever the hell is causing so much glutamate to be released. Yet another pointer to the fact that all disease originates from the gut. The skin shows the end result of what happens in the gut. We are all fleshy tubes with the topology of a donut. If you do not take care of the inside of the donut, there is no point using all manner of skin creams and sun tan lotions in a futile attempt at taking care of the outside of the donut.
My skin is gleaming, absolutely gleaming with vibrance ever since I balanced my gut bacteria using superfoods; started taking in copious amounts of spirulina, barleygrass and wheatgrass (10g of the three combined a day). Will add chlorella to that some time soon.
Kynurenic acid
From Wikipedia, the free encyclopedia
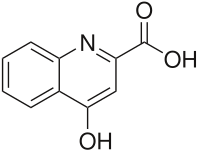
IUPAC Name: 4-hydroxyquinoline-2-carboxylic acid
Other names: Kinurenic acid, kynuronic acid, quinurenic acid, transtorine
CAS number 492-27-3
Kynurenic acid (KYNA) is a product of the normal metabolism of amino acid L-tryptophan. It has been shown that kynurenic acid possesses neuroactive activity. It acts as an antiexcitotoxic and anticonvulsant, most likely through acting as an antagonist at excitatory amino acid receptors. Because of this activity, it may influence important neurophysiological and neuropathological processes. As a result, kynurenic acid has been considered for use in therapy in certain neurobiological disorders. Conversely, increased levels of kynurenic acid have also been linked to certain pathological conditions.
Kynurenic acid was discovered in 1853 by the German chemist Justus von Liebig in dog urine, which it was apparently named after.[1]
It is formed from L-kynurenine in a reaction catalyzed by the enzyme kynurenine—oxoglutarate transaminase.
Mechanism of action
KYNA has been proposed to act on four targets:
- As an antagonist at ionotropic AMPA, NMDA and Kainate glutamate receptors in the concentration range of 0.1-2.5 mM[2]
- As a noncompetitive antagonist at the glycine site of the NMDA receptor.
- As an antagonist of the α7 nicotinic acetylcholine receptor.[3] However, recently (2011) direct recording of α7 nicotinic acetylcholine receptor currents in adult (noncultured) hippocampal interneurons by the Cooper laboratory [4] validated a 2009 study [5] that failed to find any blocking effect of kynurenic acid across a wide range of concentrations, thus suggesting that in noncultured, intact preparations from adult animals there is no effect of kynurenic acid on α7 nicotinic acetylcholine receptor currents [4] [5]
- As a ligand for the orphan G protein-coupled receptor GPR35.[6] Another tryptophan metabolite, 5-hydroxyindoleacetic acid exerts its effects via the orphan G protein-coupled receptor GPR35 [7]
Role in disease
High levels of kynurenic acid have been identified in patients suffering from tick-borne encephalitis, schizophrenia and HIV-related illnesses. In all these situations increased levels were associated with confusion and psychotic symptoms. Kynurenic acid acts in the brain as a glycine-site NMDAr antagonist, key in glutamatergic neurotransmission system, which is thought to be involved in the pathophysiology and pathogenesis of schizophrenia.
A kynurenic acid hypothesis of schizophrenia has been proposed in 2007,[8][9] based on its action on midbrain dopamine activity and NMDArs, thus linking dopamine hypothesis of schizophrenia with the glutamate hypothesis of the disease.
High levels of kynurenic acid have been identified in human urine in certain metabolic disorders, such as marked pyridoxine deficiency and deficiency/absence of kynureninase.
When researchers decreased the levels of kynurenic acid in the brains of mice, the cognition was shown to improve markedly. [10]
Edited by BLimitless, 30 January 2013 - 03:30 PM.


































































