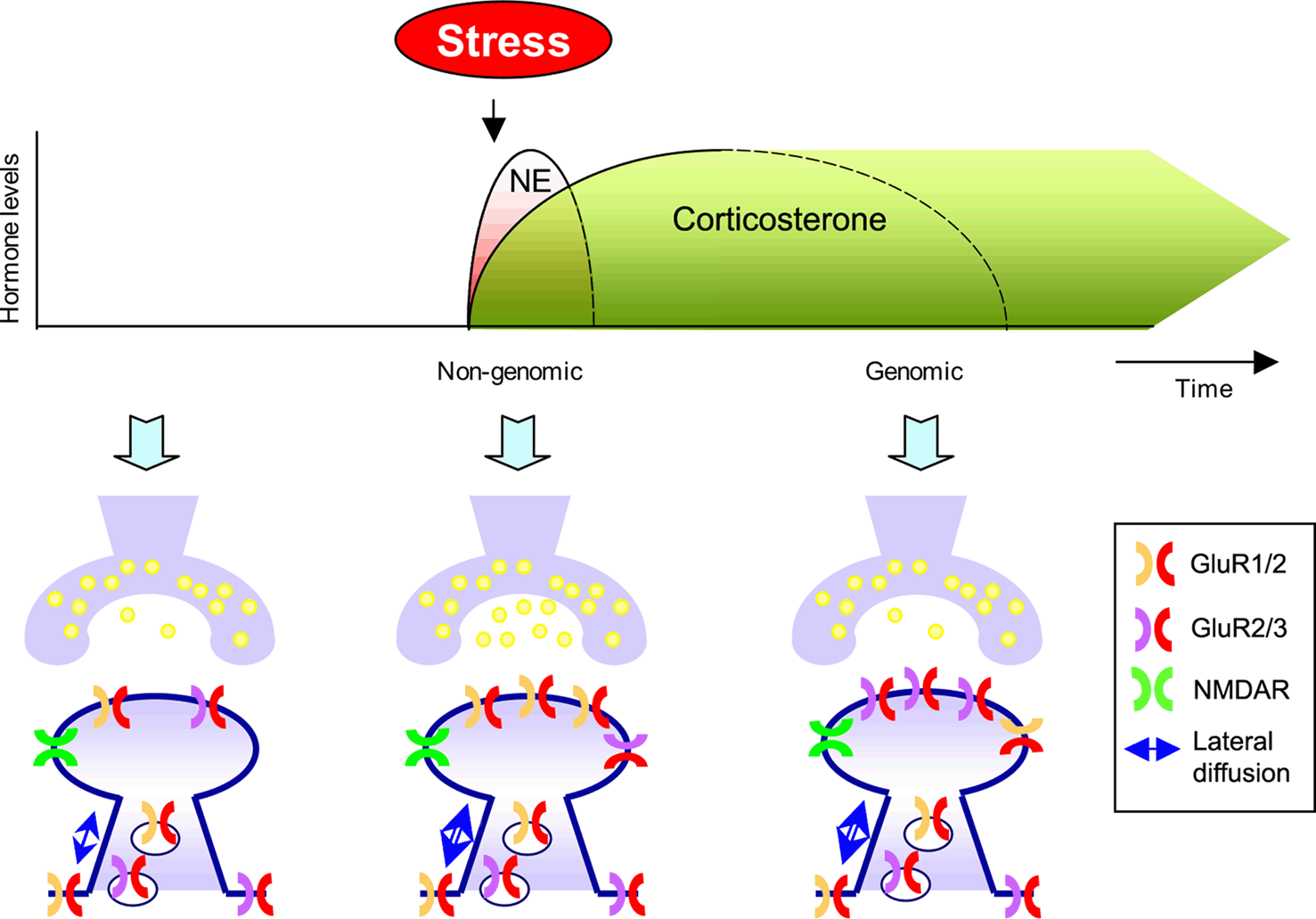
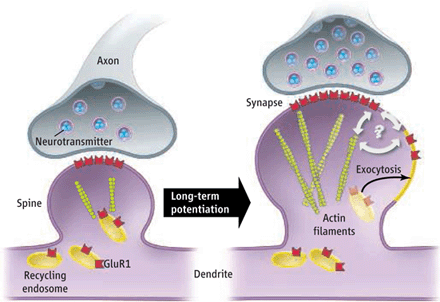
Once we have astroglia out of the way, we can recruit more AMPA receptors to the post-synaptic neuron's surface.
Activating astroglial α7-containing nicotinic acetylcholine receptors (α7-nAChRs) adds extra AMPA receptors to the post-synaptic neuron (see pic below).
Acetylcholine, nicotine, etc...activate astroglial α7-containing nicotinic acetylcholine receptors.
Galantamine is a positive allosteric modulator of α7-containing nicotinic acetylcholine receptors.
Kynurenic acid blocks the allosteric potentiating site of the alpha7AChR. See this thread for more info http://www.longecity...ad/#entry716469
Note: AMPA receptor trafficking requires ATP to cause the movement (LLLT, Methylene Blue, etc.).
http://www.ncbi.nlm....les/PMC4222677/

J Neurochem. 2013 Dec;127(5):632-43. doi: 10.1111/jnc.12436. Epub 2013 Sep 30.
Activation of α7-containing nicotinic receptors on astrocytes triggers AMPA receptor recruitment to glutamatergic synapses.
Abstract
Astrocytes, an abundant form of glia, are known to promote and modulate synaptic signaling between neurons. They also express α7-containing nicotinic acetylcholine receptors (α7-nAChRs), but the functional relevance of these receptors is unknown. We show here that stimulation of α7-nAChRs on astrocytes releases components that induce hippocampal neurons to acquire more α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors post-synaptically at glutamatergic synapses. The increase is specific in that no change is seen in synaptic NMDA receptor clusters or other markers for glutamatergic synapses, or in markers for GABAergic synapses. Moreover, the increases in AMPA receptors on the neuron surface are accompanied by increases in the frequency of spontaneous miniature synaptic currents mediated by the receptors and increases in the ratio of evoked synaptic currents mediated by AMPA versus NMDA receptors. This suggests that stimulating α7-nAChRs on astrocytes can convert 'silent' glutamatergic synapses to functional status. Astrocyte-derived thrombospondin is necessary but not sufficient for the effect, while tumor necrosis factor-α is sufficient but not necessary. The results identify astrocyte α7-nAChRs as a novel pathway through which nicotinic cholinergic signaling can promote the development of glutamatergic networks, recruiting AMPA receptors to post-synaptic sites and rendering the synapses more functional. We find that activation of nicotinic receptors on astrocytes releases a component that specifically recruits AMPA receptors to glutamatergic synapses. The recruitment appears to occur preferentially at what may be 'silent synapses', that is, synapses that have all the components required for glutamatergic transmission (including NMDA receptors) but lack sufficient AMPA receptors to generate a response. The results are unexpected and open up new possibilities for mechanisms underlying network formation and synaptic plasticity.
http://www.ncbi.nlm....pubmed/19690987
J Mol Neurosci. 2010 Jan;40(1-2):204-10. doi: 10.1007/s12031-009-9235-2. Epub 2009 Aug 19.
The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex.
Abstract
The cognitive deficits seen in schizophrenia patients are likely related to abnormal glutamatergic and cholinergic neurotransmission in the prefrontal cortex. We hypothesized that these impairments may be secondary to increased levels of the astrocyte-derived metabolite kynurenic acid (KYNA), which inhibits alpha7 nicotinic acetylcholine receptors (alpha7AChR) and may thereby reduce glutamate release. Using in vivo microdialysis in unanesthetized rats, we show here that nanomolar concentrations of KYNA, infused directly or produced in situ from its bioprecursor kynurenine, significantly decrease extracellular glutamate levels in the prefrontal cortex. This effect was prevented by the systemic administration of galantamine (3 mg/kg) but not by donepezil (2 mg/kg), indicating that KYNA blocks the allosteric potentiating site of the alpha7AChR, which recognizes galantamine but not donepezil as an agonist. In separate rats, reduction of prefrontal KYNA formation by (S)-4-ethylsulfonyl benzoylalanine, a specific inhibitor of KYNA synthesis, caused a significant elevation in extracellular glutamate levels. Jointly, our results demonstrate that fluctuations in endogenous KYNA formation bidirectionally influence cortical glutamate concentrations. These findings suggest that selective attenuation of cerebral KYNA production, by increasing glutamatergic tone, might improve cognitive function in individuals with schizophrenia.
Edited by lostfalco, 06 August 2015 - 12:50 AM.