By James P Watson with contributions and editorial assistance by Vince Giuliano
This is the third blog entry related to electronic wearable devices capable of making personal behavioral and physical parameter measurements that bear on health and wellness and likely longevity. The first blog entry was Digital health – health and fitness wearables, apps and platforms – implications for assessing health and longevity interventions – Part 1 Flux in the market. It provides a snapshot view of an important area of digital health – the rapidly changing landscape of consumer health and fitness wearables like smart watches, online and mobile health and wellness applications, and the associated emergence of software platforms that can integrate such applications together. I say snapshot because the technology and market for these devices are evolving very rapidly..
The second blog entry is Digital health – health and fitness wearables, Part 2: looking for practical stress biomarkers. It reports on initial personal experience related to identifying daily stress biomarkers that are derivable from measurements made by Vince’s Basis Peak smartwatch. Vince reports there on the results of logging data measurable by the watch for 30 days (since expanded to 47 days), analyzing it, identifying a few simple but critical stress biomarkers, and correlating the biomarker indications with known stress events he has gone through. That blog entry describes original research by Vince, defining and testing constitutional stress biomarkers. Those biomarkers are simpler and more easily, consistently, and reliably measurable using consumer wearables technology than HRV. While grounded in a history of research having to do with resting heart rates, these biomarkers are subject to validation via additional research and testing by additional people. I share Vince’s excitement about them.
This Part 3 blog entry relates to Heart Rate Variability (HRV), a well-researched biomarker for constitutional stress recovery capability. The entry is in two Sections: Section I Which is a primer about the science and usages of HRV on medicine and sports, “HeartRate Variability 101,” and Section II which is about practical HRV-measuring wearables and associated sofware. Vince and I have independently been taking HRV measurements on ourselves. Up to this time, this personal experience has been initial and inconclusive
We will probably communicate further about the theoretical bases for Vince’s biomarkers and about our practical personal experience measuring both HRV and Vince’s biomarkers in a soon-to-be-published Part 4 wearables blog entry.
Section I: Heart Rate Variability “101”
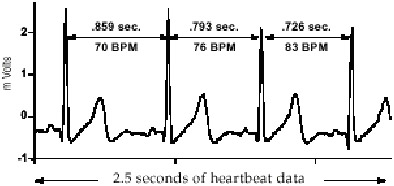
HRV refers to the fact that there are minor variations from one heart beat to another. The pulses may look the same on an emergency room monitor, but if you look carefully you will see they are slightly different, e.g. the periods between the spikes are sometimes longer or shorter and other minor differences are also likely to be present.
Not only are the pulses different, but heart rate is constantly going up or down a little. Actually this variability is a good sign because the differences between beats in a healthy person represents a complex coordination between heart actions, breathing, nervous system and other body systems. Under conditions of high stress and some pathologies these differences are suppressed and your heart beats more or less like a mechanical metronome. A rock steady beat (low HRV) is predictive of diseases and cardiovascular problems.
“Various models propose that HRV is an important indicator of both physiological resiliency and behavioral flexibility, reflecting the individual’s capacity to adapt effectively to stress and environmental demands. It has become apparent that while a large degree of instability is detrimental to efficient physiological functioning, too little variation can also be pathological. An optimal level of variability within an organism’s key regulatory systems is critical to health. This principle is aptly illustrated by a simple analogy: just as the shifting stance of a tennis player about to receive a serve may facilitate swift adaptation, in healthy individuals, the heart remains similarly responsive and resilient, primed and ready to react when needed(ref).”
Here are some summary facts about HRV:
- HRV, a measure of cardiac autonomic function, is a proxy for autonomic nervous system function, one of the fundamental physiologic systems governing organismal homeostasis.
- HRV can be measured non-invasively and safely using a diverse array of tools at little variable cost. All you need is a bluetooth communicating heart monitor strap costing as little as $50, a smartphone and computer, and to license a HRV app which could cost as little as $1.99 or be free. You may be able to get a measurement of it using your smartphone’s camera and an aoo. Section II of this blog entry lists practical alternatives.
- HRV measurement tools (including those available on personal mobile devices) already have a global footprint around the world.
- Autonomic dysfunction, as assessed by too little HRV, is associated with the panoply of aging diseases.
- Therapies that ameliorate the diseases of aging, reduce mortality rates, and improve longevity such as exercise, sleep, smoking cessation and caloric reduction have been shown also to improve HRV.
- Therapies that are known to promote health outcomes such as meditation, yoga, acupuncture and other alternative medicine treatments have been shown to improve HRV.
- Chronic stress, which has been shown to exacerbate diseases of aging, increase mortality rates, and shorten longevity, lowers HRV.
- HRV measurements via continuous fetal scalp monitoring have been used to prognosticate fetal distress and risk of mortality since 1965.
- HRV, blood pressure and heart rate statistics can be powerful predictors of all-cause mortaity
The March2015 publication Blood pressure and heart period variability ratios derived from 24-h ambulatory measurements are predictors of all-cause mortality reports “CONCLUSION: The 24-h BP to heart period variability ratios are powerful independent predictors of all-cause mortality, especially for elderly hypertensive patients with slow heart rate. The results support their interpretation as integrative indices of cardiovascular function and markers for cardiovascular dysregulation during low DBP states, with potential use in clinical practice.”
I think that HRV is a great concept, considering that it can now be measured so easily with a “ECG-quality R-to-R interval measuring, Blue tooth chest strap” and a smart phone. I describe options in Section II below
The history of HRV is very venerable. Heart Rate Variability – A Historical Perspective points out that HRV was noticed by the ancient Greeks ” ” Archigenes (1st century AD) apparently described eight characteristics of the pulse, including observations on its regularity and irregularity.”
A great deal has been learned about HRV since its discovery, and it has become an important tool of sports medicine and for sports endurance training. there has been much research on the topic Pubmed.org currently lists 18,188 research publications related to the topic.
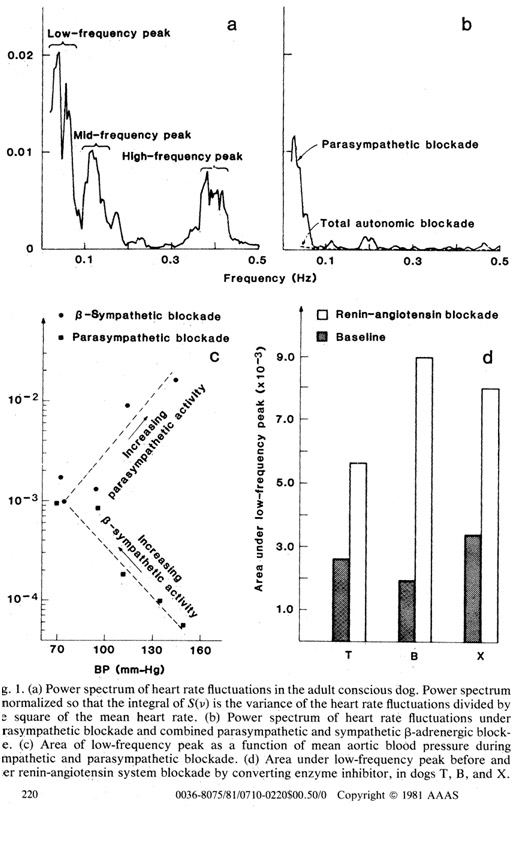
HRV analyses are based on measuring frequencies of heartbeat events, a variant of classical mathematical Fourier analysis. By looking at the relative power present in different frequency bands of a heartbeat signal, a great deal can be learned about the functioning of the autonomic nervous system and general stress. This was known back in 1981. The publication Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control then reported “Power spectrum analysis of heart rate fluctuations provides a quantitative noninvasive means of assessing the functioning of the short-term cardiovascular control systems. We show that sympathetic and parasympathetic nervous activity make frequency-specific contributions to the heart rate power spectrum, and that renin-angiotensin system activity strongly modulates the amplitude of the spectral peak located at 0.04 hertz. Our data therefore provide evidence that the renin-angiotensin system plays a significant role in short-term cardiovascular control in the time scale of seconds to minutes.”
This kind of analysis, conducted by sophisticate software, can yield much more physiologic information than a simple number which quantifies total variation.
Here are some of the things that have been learned about HRV:
- HRV spectrum: Respiratory Sinus Variation (High Frequency), Parasympathetic activity (High Frequency and Low Frequency), vs Sympathetic activity (Very low frequency)
As background, “The parasympathetic nervous system (PNS) controls homeostasis and the body at rest and is responsible for the body’s “rest and digest” function. The sympathetic nervous system (SNS) controls the body’s responses to a perceived threat and is responsible for the “fight or flight” response. The PNS and SNS are part of the ANS, or autonomic nervous system which is responsible for the involuntary functions of the human body.”: The quote is from the webpage Parasympathetic vs. Sympathetic Nervous System which compares specific aspects of the two systems.
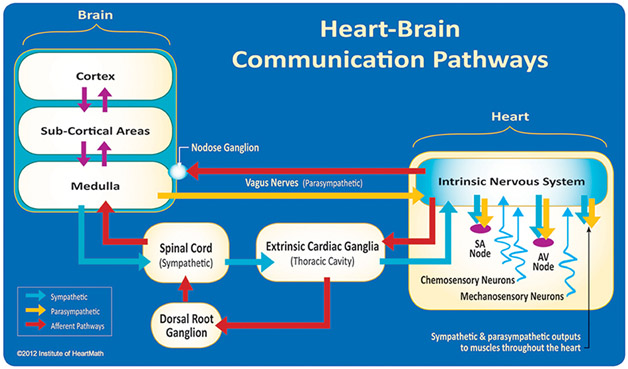
Here is a diagram of brain-heart communications that bear on HRV showing sympathetic and parasympathetic channels:
Image and legend source: A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability, “Figure 3. The neural communication pathways interacting between the heart and the brain are responsible for the generation of HRV. The intrinsic cardiac nervous system integrates information from the extrinsic nervous system and from the sensory neurites within the heart. The extrinsic cardiac ganglia located in the thoracic cavity have connections to the lungs and esophagus and are indirectly connected via the spinal cord to many other organs such as the skin and arteries. The vagus nerve (parasympathetic) primarily consists of afferent (flowing to the brain) fibers which connect to the medulla, after passing through the nodose ganglion. Credit: Institute of HeartMath.”
HRV power spectrum analysis can differentiate between HRV changes that are due to breathing (called Respiratory Sinus Variation) and HRV changes that are due to the autonomic system (ANS).
“The normal variability in heart rate is due to the synergistic action of two branches of the ANS (the sympathetic and parasympathetic branches), which act in balance through neural, mechanical, humoral and other physiological mechanisms to maintain cardiovascular parameters in their optimal ranges and to permit appropriate reactions to changing external or internal conditions. In a healthy individual, thus, the heart rate estimated at any given time represents the net effect of the parasympathetic (vagus) nerves, which slow heart rate, and the sympathetic nerves, which accelerate it. These changes are influenced by emotions, thoughts and physical exercise. Our changing heart rhythms affect not only the heart but indirectly also the brain’s ability to process information, including decision-making, problem-solving and creativity. They also directly affect how we feel. Thus, the study of heart rate variability is a powerful, objective and noninvasive tool to explore the dynamic interactions between physiological, mental, emotional and behavioral processes(ref).”
- Very low frequency variations (power) in HRV are primary sympathetic nervous system activity and include frequencies in the range of 0.04 – 0.15 Hz
- Autonomic system variations in HRV include both sympathetic and parasympathetic activity, but there is a lot of overlap in the spectrum between these two divisions of the autonomic system
- High frequency (HF) variations (power) are due to respiratory variations and are mediated by the parasympathetic system. HF are frequencies in the range of 0.25 Hz. HF power can be improved with exercise and deep breathing. Because HF power (respiratory changes) is driven by parasympathetic tone, HF power is dramatically increased in endurance athletes who have a resting bradycardia (low resting heart rate).
- Low frequency variations (power) in HRV are due primarily to the parasympathetic system and include frequencies in the range of 0.15 – 0.4 Hz.
- However, less sophisticated HRV analysis systems lump VLF and LF into one power spectrum. When this is done, they typically do a ratio of LF to HF (LF/HF), which is normally about 3.6 + 0.7
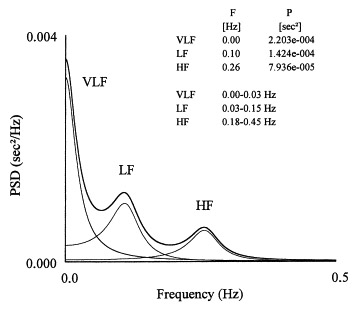
Here is a graph of these spectrums:
“Figure 2. Power spectrum of HRV (PSD = power spectral density).” Graph and legend from Heart Rate Variability Summary prepared by Ichiro Kawachi in collaboration with the Allostatic Load Working Group. Last revised 1997. “The approach uses Fourier transforms. The HRV spectrum contains two major components: the high frequency (0.18-0.4 Hz) component, which is synchronous with respiration and is identical to RSA. The second is a low frequency (0.04 to 0.15 Hz) component that appears to be mediated by both the vagus and cardiac sympathetic nerves. The power of spectral components is the area below the relevant frequencies presented in absolute units (square milliseconds). The total power of a signal, integrated over all frequencies, is equal to the variance of the entire signal. Some investigators have used the ratio of the low-to-high frequency spectra as an index of parasympathetic-sympathetic balance; however, this remains controversial because of our lack of complete understanding of the low frequency component (which seems to be affected by centrally generated brainstem rhythms, baroreceptor feedback influences, as well as both sympathetic and vagal input).”
Another diagram is
Other publications describing the overall HRV approach include:
- Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control 1981
- Heart rate variability in athletes 2003
- Sympathetic control of short-term heart rate variability and its pharmacological modulation 2007
- Low-frequency oscillations in arterial pressure and heart rate: a simple computer model 1989
- Autonomic differences between athletes and nonathletes: spectral analysis approach 1997
- Supine vs. Standing HRV Measurement: Is one better than the other? 2012
- Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog 1986
As you can see from the references, HRV has been of great interest in the sports medicine and training fields.
Practical conclusions:
- We need a sensor and software that can tell us the “power spectrum” of HRV, including the HF power (0.25 Hz), LF power (0.15-0.4 Hz) and hopefully the very LF power (0.04-0.15 Hz)
- The ratio of LF to HF is normally about 3.7 and ideally, we need a software program that computes this as well.
- We want to decrease sympathetic tone (VLF or LF), increase parasympathetic tone (HF and LF), slow down heart rate, and increase deep breathing (HF) to improve HRV. These are not going to be easy to do in a rat or a mouse. But the good news is that if we are healthy, HRV measurement can guide us for doing them for ourselves
Ideally, we will simultaneously use a sensor that senses respiration, since this is such an important aspect of HRV
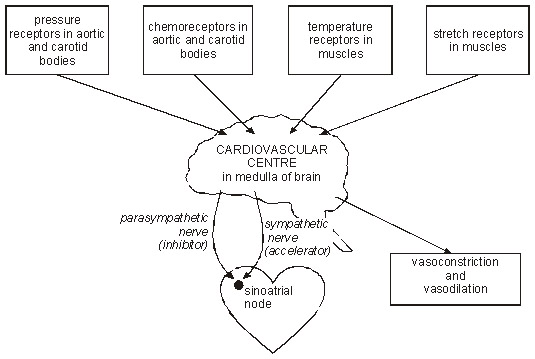
- HRV Physiology: What “drives” Heart Rate Variability?
There are several anatomic structures in the brain, the brainstem, the aorta (pressure and chemoreceptors), carotid artery (pressure and chemoreceptors in the carotid body), and the heart (SA node) that “drive” heart rate variability. Stretch receptors in the muscles also “drive” HRV.
References:
Reference for diagram: Supine vs. Standing HRV Measurement: Is one better than the other?
HRV changes with Posture: Supine vs Sitting vs Standing position
HRV dramatically changes with body position – this is something we will NOT have to deal with in mice or rats (they don’t walk upright)
In the supine position, heart rate and blood pressure are low. In the supine position, sympathetic activity is low and parasympathetic activity is high.
Just changing from the supine to sitting position changes these parameters somewhat, which can be measured in the power spectrum of HRV (i.e. LF vs HF). In the supine position, the HF power spectrum is high. In the sitting position, the HF power spectrum of HRV decreases from 25% to 6.2% (p < 0.005). In the supine position, the LF power spectrum is low. In the sitting position, the LF power spectrum of HRV increases. One practical implication is that if you are older with a relatively lower HF power spectrum, you might get better HRV measurements while supine.
With standing, there is a dramatic withdrawal of parasympathetic activity and an increase in sympathetic activity. This is not surprising, considering that these autonomic refexes are necessary to maintain blood pressure to the brain, in light of the standing position (i.e. gravity)
What is surprising is that endurance exercise training increased HRV in the HF power spectrum, but did not change HRV in the HF power spectrum with subjects in the supine position (Kiviniemi, 2007).
Conclusions:
- Because body position makes such a big difference on the HRV power spectrum and overall HRV values, measuring HRV in the same position is key to avoiding false positives and false negatives and getting consistent day-to-day HRV measurements.
- Standing position measurements may be a better reflection of the value of physical exercise if you are an athlete with a low heart rate. If you are older or have a higher heart rate, on the other hand, the supine position might be better.
- Postural changes will not be a factor in mice or rats. This is why the field is “wide open”, when it comes to doing HRV work in four legged creatures that do not walk on two legs
References:
- Supine vs. Standing HRV Measurement: Is one better than the other?
- Standing vs. Supine ithlete HRV Measurement 2012
- Effects of high altitude acclimatization on heart rate variability in resting humans 1996.
- Autonomic nervous control of heart rate at altitude (5050 m) 1994
- HTN, CHF, and MI => increased sympathetic afferent output: Reducing autonomic (sympathetic) afferents from the brain, via the cardiac sympathetic nerves
Patients with hypertension (HTN) and chronic heart failure (CHF) have reduced HRV due to many factors. After a myocardial infarction (MI), there is also a decrease in HRV. HRV can predict mortality after an MI and in patients with CHF.
- In these patients, there is strong evidence that reduced parasympathetic activity contributes to the decrease in HRV with CHF
- In these patients, there is strong evidence that reducing cardiac sympathetic nerve activity increases HRV
- Major factors are salt intake, Angiotensin II, decreased endothelial NO, and increased sympathetic afferent output
I do not think HRV would improve that much by consuming plant polyphenols, but reducing the output from the CNS to the cardiac sympathetic nerves (CSNs) should make a big difference. CSN output is a separate effect from respiratory sinus arrhythmia (RSA). ” Respiratory sinus arrhythmia (RSA) is heart rate variability in synchrony with respiration, by which the R-R interval on an ECG is shortened during inspiration and prolonged during expiration. Although RSA has been used as an index of cardiac vagal function, it is also a physiologic phenomenon reflecting respiratory-circulatory interactions universally observed among vertebrates(ref).”
RSA is involved in the high frequency spectrum of HRV, whereas CNS activity is involved with the slow frequency HRV with a period of about 10 seconds.
Obviously exercise and sleep will help, but I do not think that yoga, meditation, or Tai Chi is very feasible in mice or rats. We may be able to reduce CNS output with an RF interference electrode that could be “trained” to inhibit CNS output. In rodents with aging that have low eNOS activity and low nitric oxide levels in their blood vessels, chemical sympathectomy with 6-hydroxy dopamine restores HRV. We may want to do this as well.
References:
- Sympathetic control of short-term heart rate variability and its pharmacological modulation 2007
- Nitric oxide and cardiac parasympathetic control in human heart failure
- Cardiac sympathetic nerve stimulation does not attenuate dynamic vagal control of heart rate via α-adrenergic mechanism 2004
- Dynamic characteristics of heart rate control by the autonomic nervous system in rats 2010
- Chemical sympathectomy restores baroreceptor-heart rate reflex and heart rate variability in rats with chronic nitric oxide deficiency 2014
- Sympathetic predominance an essential hypertension: a study employing spectral analysis of heart rate variability 1988
- Heart rate variability as an index of sympathovagal interaction after acute myocardial infarction 1987
Conclusions:
- Controlling HTN is clearly important for keeping HRV high.
- There may be a possibility of doing a “chemical sympathectomy” with 6-hydroxy dopamine in our “reference mammals.”
- Reducing Renin-angiotensin signaling
There is some evidence that reducing Renin Angiotensin II signaling (RAS) has a beneficial effect on HRV, This primarily improves low frequency power of the HRV power spectrum, Many studies of sleep have shown that Angiotensin II levels decrease at night in a circadian fashion (no surprise!).
At night, there is an increase in baroreceptor reflex sensitivity, which is the highest during dreaming. “Baroreceptors (or archaically, pressoreceptors or baroceptors) are sensors located in the blood vessels of all vertebrate animals. They sense the blood pressure and relay the information to the brain, so that a proper blood pressure can be maintained(ref).” With CHF, the circadian changes in HR and the baroreceptor reflex (BRS) is blunted. This explains part of the mortality of CHF that is independent from LVEF
Angiotensin Type I receptors are the main way that this harmful effect of Ang II is mediated. This may be why the ATR1 receptor blockers seem to have a longevity effect. See our blog entry ACE and Angiotensin II: The “Double Agents” that Play Multiple Roles in the Molecular Story of Life.
References:
- Angiotensin II disproportionally attenuates dynamic vagal and sympathetic heart rate controls 2008
- Reflex Regulation of Arterial Pressure during Sleep in Man – A Quantitative Method of Assessing Baroreflex Sensitivity 1969
- Disruption of cardiovascular circadian rhythms in mice post myocardial infarction: relationship with central angiotensin II receptor expression 2014
- Abnormal baroreflex function is dissociated from central angiotensin II receptor expression in chronic heart failure 2012
- Role of AT1 receptors in the central control of sympathetic vasomotor function 1996
- Effect of losartan, an angiotensin II type 1 receptor antagonist on cardiac autonomic functions of rats during acute and chronic inhibition of nitric oxide synthesis 2012
Here is a diagram of what happens with Renin-Angiotensin system blockade and HRV.
Reference for diagram: Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control
Conclusion: Blockade of the Renin-Angiotensin system increases the area under the curve of the low frequency HRV power spectrum. Using ATR1 blockers appears to be the best way to do this.
- Exercise and HRV – HF power increases, LF power decreases in endurance athletes. Supine position with exercise.
Endurance/Aerobic exercise
Endurance, aerobic exercises increases HRV in the high frequency (HF) spectrum (respiratory sinus arrthymia) but actually lowers the low frequency (LF) power spectrum. Endurance athletes therefore have higher HF power spectrum and lower LF power spectrum
This drop in LF power spectrum may represent an imbalance between parasympathetic activity and sympathetic activity, due to the resting bradycardia seen in endurance athletes. This resting bradycardia is due to increase in parasympathetic outflow.
Weight lifting exercise (supine position)
Because the sympathetic system signaling is dramatically decreased when the body is in the supine position, weight lifters working out in on a bench in the supine position display a dramatic increase in HRV in the low frequency (LF) power spectrum. This means that there is a decrease in sympathetic activity when in the supine position.
References:
- Assessment of training-induced autonomic adaptations in athletes with spectral analysis of cardiovascular variability signals 1995
- Heart rate variability in athletes 2003
- Autonomic differences between athletes and nonathletes: spectral analysis approach 1997
- Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions 2003
- Sleep and HRV -
Sleep has a beneficial effect on HRV. At night, Angiotensin II levels go down, increasing HRV. Baroreceptor reflex sensitivity goes up at night. It is the highest during dreaming – REM sleep.
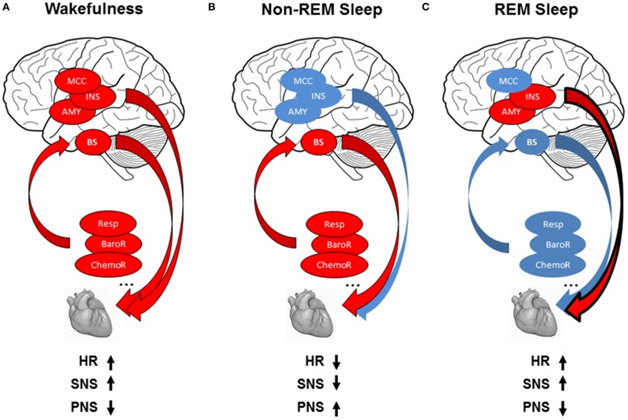
Here is a diagram showing typical behaviors of heart rate, the parasympathetic and the sympathetic nervous systems during wakefulness, non-REM and REM sleep.
Image and legend source “(A) Modulation of cardiac activity during wakefulness: reflex loops [baroreflex (BaroR), respiration (Resp), chemoreflex (ChemoR)] including brainstem centers (BS) and central autonomic network including midcingulate cortex (MCC), insula (INS), amygdala (AMY) contribute to cardiac activity, leading to increased heart rate (HR), increased sympathetic activity (SNS), and decreased parasympathetic activity (PNS). (B) Modulation of cardiac activity during non-REMS: The drop in brain activity, with predominant contribution of reflex loops on ANS activity, leads to decreased HR, with parasympathetic predominance, and decrease in sympathetic modulation. © Modulation of cardiac activity during REMS: autonomic cardiac regulation is shared between central control in relation with the insula and amygdala and homeostatic control of the cardiovascular system by reflex loops, leading to decreased HR with sympathetic predominance and decreased parasympathetic activity. Red circles indicate increase and blue circles decrease in autonomic cardiac activity.“
Reference: Reflex Regulation of Arterial Pressure during Sleep in Man 1969
HRV has been suggested to be an excellent tool for the analysis of sleep and for distinguishing between healthy and pathological sleep. See these additional references.
Conclusions:
- Baroreceptor sensitivity can be rapidly “reset” at night. This is very important for controlling blood pressure
- HRV results are likely to be different during during REM and non-REM sleep
- HRV can be a useful tool for distinguishing between sleep disorders.
- Oxidative stress and HRV
Rodents have accelerated telomere shortening that is NOT due to replicative senescence, This means they probably have a lot of oxidative stress. Although I could not find any articles specifically linking oxidative stress to HRV, I found some articles that showed that ROS-producing chemotherapy (alkylating agents) decreased HRV
Polyphenols and MitoQ may help some, but I think the following would be better:
- Bucky Balls – See our blog entries on C60 fullerenes(ref)(ref),
- Methylene Blue(ref)(ref)(ref), and
- a few of the other potent mitochondrial-specific antioxidants like MitoQ that change the membrane potential across the inner mitochondrial membrane.
I speculate that these could potentially make a huge difference in HRV
References:
- The influence of oxazaphosphorines alkylating agents on autonomic nervous system activity in rat experimental cystitis model 2013
- Cardiac autonomic function in acutely nitric oxide deficient hypertensive rats: role of the sympathetic nervous system and oxidative stress 2011
- Gut bacteria/Celiac disease
Some fascinating information is coming out about the effect of wheat intake in patients with celiac disease. Patients with celiac disease have a much lower HRV than controls. Also, patients with celiac disease have less HRV with deep breathing.
- 20% of the patients with celiac disease had parasympathetic dominance, whereas
- 36% of the patients with celiac disease had sympathetic dominance
- 44% of the patients did not show parasympathetic or sympathetic dominance
Measurements can show decreases in HRV with all three of these groups of celiac disease patients.
Reference: Disturbances of autonomic nervous system activity and diminished response to stress in patients with celiac disease 2014.
- Stress and Glucocorticoids - glucocorticoids, epinephrine (from adrenal gland), and norepinephrine (from sympathetic nerves) all decrease HRV
Stress reduces HRV by several hormones and neurotransmitters, including glucocorticoids, epinephrine, and norepinephrine. Glucocorticoids (cortisol, synthetic steroids, etc.) decrease heart rate variability. Glucocorticoids also increase systolic blood pressure. The mediator of this is impaired hydrogen sulfide signaling.
References:
- Vascular Disease: Biology and Clinical Science — Session Title: Microcirculation and Cerebral/Coronary/Peripheral Circulation II 2014
- Glucocorticoids modulate baroreflex control of heart rate in conscious normotensive rats
High sodium diet - This decreases HRV
A high sodium diet decreases HRV, The exact mechanism is not clear, but it probably involves blood pressure changes and changes in nitric oxide (NO) synthesis.
Reference: Contribution of nitric oxide to arterial pressure and heart rate variability in rats submitted to high-sodium intake 2001
- Gasotransmitters – Nitric oxide (NO), Hydrogen Sulfide (H2S), and Carbon Monoxide (CO)
Much has been learned about HRV from patients with congestive heart failure (CHF) and patients with coronary artery disease (CAD). In CHF, there is a dramatic decrease in heart rate variability (HRV) and an increase in low frequency systolic blood pressure variability (SBPV). CHF also results in increases in breath interval variability (i.e. snoring, sighing, etc.), and an increase in apneic episodes (like in obstructive sleep apnea).
These effects in CHF are thought to be mediated by the carotid body (CB), which senses oxygen levels. Hypoxia causes an increase in CB signaling. The molecular mediators of these changes in CHF include sympathetic dysregulation, renin-angiotensin dysregulation, increased atrial naturetic peptide (ANP), increased brain naturetic peptide (BNP), and increase in Hypothalamic-pituitary-adrenal axis hormones (cortisol, epinephrin, aldosterone).
However, the most recent molecular mediator discovery in CHF is the gasotransmitters – they play a major role in the pathogenesis of CHF. These gasotransmiters and their dysregulation plays a major role in the decrease of HRV that occurs with these diseases.
Nitric Oxide is “good”, but NO dysregulation is “bad“- acute exogenous NO administration is ‘good”, but chronic exogenous NO administration results in “nitrate tolerance”.
Inhibition of endogenous NO production decreases HRV in some circumstances. In others, inhibition of endogenous NO production increases HRV
Nitric oxide has “good” and “bad” effects that are very hard to understand. Nitric oxide is thought to play an important role in the tonic control of carotid body (CB) chemosensitivity. Both eNOS and nNOS are present in the CB. NO inhibits CB activity by suppressing the CB chemoreceptors during normoxic periods.
Basal NO production, eNOS and nNOS expressio in the CB are depressed with CHF. Thus there is no inhibition of the CB in CHF, due to a decrease in NO.
Part of the Paradox of NO is due to the differences between endogenous production of NO and exogenous production of NO. In addition, part of the paradox is due to the differences between “acute” exogenous NO administration vs “chronic” exogenous NO administration.
- Acute NO administration reduces sympathetic activity and increases vagal outflow
- Chronic NO administration results in a loss of these effects and the development of “nitrite tolerance”
The easiest way to understand this is that “nitric oxide acts at distinct levels in the autonomic system to control cardiac rate, with opposing effects at different sites
In general, endothelial nitric oxide has mostly “good effects.” For instance, neuronal nitric oxide inhibition decreases HRV 3-fold by inhibiting vagal stimulation, whereas increased neuronal NO increases HRV. For instance, endothelial Nitric oxide synthase 3 increases NO after an MI and this increases survival from an MI – this also increases HRV
- Nitric oxide increases in response to a high sodium diet – this reduces HRV.
- With CAD, nitric oxide levels from eNOS fall.
References
- Contribution of nitric oxide to arterial pressure and heart rate variability in rats submitted to high-sodium intake 2001
- Endothelial Nitric Oxide Synthase and Heart Rate 2002
- Effects of nitroglycerin treatment on baroreflex sensitivity and short-term heart rate variability in humans 2002
- Nitric oxide and autonomic control of heart rate: a question of specificity 2002
- Nitric oxide and cardiac parasympathetic control in human heart failure 2002
- Nitric oxide synthases in vagal neurons are crucial for the regulation of heart rate in awake dogs 2001
- Role of the Carotid Body in the Pathophysiology of Heart Failure 2013
Conclusions: Administration of exogenous NO (acutely for a short period of time) may increase HRV, whereas chronic administration would not. Administration of an exogenous NO donor (such as L-arginine) would also increase HRV, but probably not continuously on a permanent basis.
Hydrogen Sulfide is “good”, but dysregulation of H2S is “bad” – H2S is involved with carotid body chemoreceptor sensitivity and with breathing control
Inhibition of hydrogen sulfide restores normal breathing, but increased hydrogen sulfide increases survival after an MI. Hydrogen sulfide also increases antioxidant gene expression via an Nrf2-mediated pathway. H2S product by the enzyme CSE is not decreased in CHF – CSE inhibition with CHF reduces carotid body afferent responsiveness
There are a lot of puzzling paradoxes about H2S that I do not understand.
- Hydrogen sulfide signaling upregulates antioxidant gene expression by the transcription factor, Nrf2 – this is a “good thing”
- Hydrogen sulfide also increases survival after an MI via a nitric oxide synthase 3 dependent mechanism – this is a “good thing”
- However, H2S production by the carotid body chemoreceptors can also have “bad consequences”
- H2S is known to be an important signaling molecule in the carotid body (CB). H2S is synthesized by cystathionine gamma-lyase (CSE) in the CB
- Carbon monoxide (CO) inhibits CSE, thereby increasing HRV.
- Exogenous CSE inhibitors such as PAG also increase HRV
- Evidently in response to hypoxia, hydrogen sulfide signaling reduces HRV and causes abnormal breathing (apnea and increased breath intervals)
- Increased hydrogen sulfide signaling from the carotid body hypoxia sensor is part of the pathology of CHF.
- These effects in CHF are thought to be mediated by the carotid body (CD), which senses oxygen levels.
- In CHF, there is an increase in carotid body (CB) chemoreceptor outflow due to hypoxia. The “signaler” is the gasotransmitter, hydrogen sulfide
- Thus CHF results in increased CB hydrogen sulfide synthesis, which reduces HRV. increases breath interval variability (BIR), and increases apneic episodes
- Inhibitors of hydrogen sulfide synthesis (by cystathionine gamma-lyase) like the drug di-propargylglycine (PAG) inhibit H2S synthesis by the CB. In rodents, this reduced the apnea index by 90%, reduced breath interval variability by 40-60%,
Hydrogen sulfide signaling is a major reason why HRV decreases with aging.
References”
- Vascular Disease: Biology and Clinical Science — Session Title: Redox Signaling and Oxidative Stress Abstract 17514: Hydrogen Sulfide Levels and NRF2 Activity are Attenuated in the Setting of Critical Limb Ischemia (CLI)
- Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3 dependent mechanism in mice 2010
- Chemical sympathectomy restores baroreceptor-heart rate reflex and heart rate variability in rats with chronic nitric oxide deficiency 2014
- Baroreceptor reflex function in rats submitted to chronic inhibition of nitric oxide synthesis 1994
- Nitric oxide synthesis blockade reduced the baroreflex sensitivity in trained rats 2009
- Inhibition of hydrogen sulfide restores normal breathing stability and improves autonomic control during experimental heart failure 2013
- Role of the Carotid Body in the Pathophysiology of Heart Failure 2014
Conclusion: Since CSE is not down regulated in CHF, hydrogen sulfide probably plays a supportive role, rather than a causal role in CHF. I am not sure if we want to inhibit it or activate hydrogen sulfide signaling. In CHF, we probably want to inhibit it. Under normal conditions, I am not sure. If we want to improve breathing, however, we will need to inhibit H2S production.
If we want to increase Nrf2 gene expression, however, we will want to activate H2S production or give exogenous H2S. To improve HRV, we may need to use a H2S synthesis inhibitor like PAG to down regulating H2S signaling from the carotid body
Carbon Monoxide is “good”, but dysregulation or too much CO is “bad” - CO production in the CB is due to HO-2 gene expression and is reduced in CHF
Carbon monoxide (CO) is the 3rd and last gasotransmitter to be discovered. CO is very important to health. The first case of a human to have a gene mutation in HO-1 (heme oxygenase-1, the enzyme that synthesizes CO) died at age 6. HO-1 gene knock-out models in mice and rats also die at a young age. It came as a surprise to many that the body synthesizes CO – to be exact, it makes 16.4 micro moles per hour of CO – all from heme breakdown. The total amount of CO produced per day is 12cc (see ref below)
In the pulmonary artery, CO relaxes the pulmonary vasculature under normoxic conditions. In the carotid artery, CO regulates (decreases) the sensitivity of the carotid body to hypoxia. However, CO plays a much greater systemic role in the body – CO is synthesized during heme breakdown by heme oxygenase 1 (HO-1), heme oxygenase 2 (HO-2), and heme oxygenase 3 (HO-3).
HO-1 is the inducible form of the enzyme and is primarily found in the spleen, but is also expressed in other tissues in lower amounts. The spleen is the only organ where HO-1 overpowers HO-2. HO-1 is NOT expressed in the carotid body (CB). HO-2 is the constitutively activated form that is mostly expressed in the brain and testes – it is expressed in the carotid body (CB). HO-3 is another constitutive form of the enzyme that has only been found in rat tissues so far.
With CHF, the expression of the constitutively activated HO-2 in the CB is decreased. This means there is less CO produced by the CB with CHF.
References:
- Role of the Carotid Body in the Pathophysiology of Heart Failure 2013
- Carbon Monoxide: Endogenous Production, Physiological Functions, and Pharmacological Applications 2005
- Disturbed heart rate variability: a dose-dependent response to elevated nitric oxide and carbon monoxide in exhaled breath 2013
- Chapter 2 The Role of Carbon Monoxide as a Gasotransmitter in Cardiovascular and Metabolic Regulation
Conclusions:
- Exogenous administration of low dose CO (acutely only) may have a beneficial effect on HRV, since this inhibits CSE and thereby H2S production
- Exogenous administration of low dose CO (acutely) also induces the enzyme that makes endogenous CO, HO-1.
- For this reason, exogenous CO administration should have both a direct and indirectly beneficial effect on HRV, but no one has measured this yet
- Chronic Hypoxia, Rapid ascent to altitude, High Altitude Acclimatization, and High Altitude Training in Athletes
Chronic hypoxia does not affect heart rate that much, but it has a dramatic negative effect on HRV. This is why obstructive sleep apnea and COPD are so bad for health.
Rapid ascent to altitude decreased HRV in both the LF and HF power spectrums. In these individuals who ascended rapidly to altitude, 48% of them developed acute mountain sickness (AMS). This decrease in HRV happened in individuals who developed AMS and in those who did not develop AMS (no difference).
Acclimatization has been reported to have conflicting results on HRV. One study found that acclimatization at low altitudes (2,000-3,000 meters) and high altitude (5,000 meters) did not modify HRV in any statistically significant fashion. Another study done in tourists at 2,700 meters and at 3,700 meters showed a decrease in HRV at both altitudes. This decrease in HRV occurred in both the HF and LF power spectrums. At 3,700 meters, the sympathetic system was dominant over the parasympathetic system. Another study done in high altitude mountaineers found that at sea level, postural changes are mainly controlled by increases in sympathetic tone with sitting and standing, whereas at high altitude, postural changes are mainly controlled by a decrease in parasympathetic activity and not an increase in sympathetic activity (5,000 meters). However, high Altitude does not necessarily impair HRV, even though there is continuing exposure to low oxygen tension.
In trained Andean participants, completing a marathon at 4,220 meters elevation transiently increases sympathetic predominance of HRV after the marathon for less than 1 day. Then their HRV returns to a healthy, baseline where parasympathetic tone dominates over sympathetic outflow.
References:
- Heart rate variability in rats with chronic hypoxic pulmonary hypertension 2006
- Autonomic adaptations in andean trained participants to a 4220-m altitude marathon 2005
- Effect of rapid ascent to high altitude on autonomic cardiovascular modulation 2008
- Effects of high altitude acclimatization on heart rate variability in resting humans 1995
- Alterations in autonomic nervous control of heart rate among tourists at 2700 and 3700 m above sea level 2001
- Autonomic nervous control of heart rate at altitude (5050 m) 1994
Conclusion: I do not see any way how manipulating atmospheric pressure by reducing it would help HRV
- Air pollution
Air pollution has been shown to decrease HRV (that’s bad!) The best study on this was done at Harvard’s School of Public Health. They found that pollution in general reduced HRV and Blood Pressure Variability (BPV), but that ozone (O3) and moving averages of particulate pollution in the air (PM2.5) were most associated with a decrease in HRV and BPV. The decrease in standard deviation of normal-to-normal HRV (SDNN) and low frequency power spectrum (LF) were greatest in diabetics.
FReference: Effects of air pollution on heart rate variability: the VA Normative Aging Study. A new study dated January 15 2015
Section II: Consumer HRV Hardware and Software 101
The marketplace is offering a wide variety of consumer hardware and software options for measuring HRV, particularly newer options where bluetooth measuring devices link to smartphones with apps that link to personal HRV measurements databases on the web. We list some of these options here.
A. HRV Hardware
Chest Strap systems:
Chest straps are the most well-developed devices that give “ECG-quality R-to-R interval measurements” and wirelessly communicate to your smart phone. The trend is to go to Bluetooth straps, rather than the old ANT+ standard that the fitness HR straps used in the past. A new Bluetooth standard used in some belts, Bluetooth® 4.1, the latest standard, is a low energy one that increases battery life dramatically. This low energy Bluetooth allows you to “sync” your HR strap to a smart phone or tablet PC. Here is a list of several Bluetooth straps and a few ANT+ straps that can be used for HRV.
Polar H7 Bluetooth strap – Bluetooth 4 low energy
hardware compatibility – compatible with either iPhones (IOS) or Android phones (Android 4.3 or greater)
- transmits the data in real time to a phone or to a Polar watch (like the M400)
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: 350 hours – need to unsnap device from belt after use to get this.
- cost – $50-70
- nice feature – can wirelessly connect to a lot of gym equipment.
- Both Vince and I have been using this sensor for our personal HRV measurements.
Wahoo TICKR heart rate monitor strap – this is the entry level Wahoo strap – it has both ANT+ and Bluetooth wireless links
- phone compatibility – compatible with either IOS (iPhone 4S or later) or Android phones (Android 4.3 or later version)
- software compatibility – compatible with Apple Healthkit and over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: 350 hrs
- cost – $58-70
- nice feature – has a free 8 week Wahoo Fitness Burn and Burst Training app
Wahoo TICKR Run strap – this is Wahoo’s more advance version that has an accelerometer built into it – its wireless links also have both ANT+ and Bluetooth 4.0
- phone compatibility – compatible with either IOS (iPhone 4S or later) or Android phones (Android 4.3 or later version)
- software compatibility – compatible with Apple Healthkit and over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – yes
- battery life: 350 hrs
- cost – $68-80
- nice feature – has a Wahoo Running Smoothness Metrics program/feature
Head-to-head review of Polar H7 and Wahoo TICKR straps – BATTLE! – Wahoo Fitness TICKR RUN Heart Rate Monitor vs Polar H7 Heart Rate Sensor + Polar Stride Sensor
60beat BLUE strap – Bluetooth 4 low energy
- hardware compatibility – compatible with either iPhones (IOS) or Android phones (Android 4.3 or greater)
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- cost – $35-60
- nice feature – comes with several free apps called MapMyRun, RunMeter, Endomondo, LogYourRun, and RunKeeper
Cardiosport Bluetooth SMART strap – Bluetooth 4.0 low energy
- hardware compatibility – compatible with either iPhones (IOS)
- not compatible with Android phones
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- cost – $35-60
- nice feature – comes with several free apps called MapMyRun, RunMeter, Endomondo, LogYourRun, and RunKeeper
- Review of this strap: ithlete Cardiosport Bluetooth SMART Heart Rate Monitor Strap – In Depth Review
smartLABhBeat HRM strap – Bluetooth 4.0 low energy with Bluetooth Smart technology
- phone compatibility – compatible with iPhones or iPads
- Works with several APPs that support HRM on iOS, Android and Windows mobile devices
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: rechargeable battery with 26 hrs battery life
- cost – $39-40 (big price drop recently)
- nice feature – cheap!
Zephr HxM Smart HRM strap – Bluetooth 4.0 low energy (no rechargeable battery on this model)
- phone compatibility – compatible with Android phones (Android 4.3 or later version), and with Windows 8 phone devices
- not compatible with iPhones or iPads
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: rechargeable battery with 26 hrs battery life
- cost – $50-60
- nice feature – waterproof up to 1 meter
Zephr HxM BT strap – Bluetooth 4.0 low energy and a rechargeable battery
- phone compatibility – compatible with Android phones (Android 4.3 or later version) and Windows phones
- no compatible with iPhones or iPads
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: rechargeable battery with 26 hrs battery life
- cost – $60-80
- nice feature – charging cradle to recharge the battery (most straps do not have rechargeable batteries)
- review of this strap: Keep the beat with the Zephyr hxm bluetooth heart rate monitor
4iii Innovations V100 viiiva strap – ANT+ and Bluetooth Smart wireless linkages
- phone compatibility – compatible with iPhones
- not compatible with Android phones
- can send real time info to ANT+ watches or bicycle computers
- software compatibility – compatible with any iPhone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: 200 hrs
- cost – $80
- pros – has both ANT+ and Bluetooth technology
- cons – not compatible with Polar devices and doesn’t work with legacy Bluetooth standard
- online review of this strap: 4iiii’s Viiiiva ANT+ to Bluetooth Smart Bridge & Heart Rate Strap In-Depth Review
Pear Sports Mobile Training Intelligence strap – This one of those that can be used with either iPhone or Android phones
- Bluetooth 4.0 low energy with Bluetooth Smart technology
- phone compatibility – compatible with iPhones, iPods, and iPads (4 or later model)
- compatible with Android phones
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: 400 hrs
- cost – $80-100
- optional foot pod
- Marketed as a comprehensive training system. Real-time coaching while exercising is via heart rate feedback.
- Nice features – 1. Realtime Audio training coaching, 2. comes with PEAR “Stride” earphones with Earlock ear pieces
Online reviews:
- Product Review: PEAR Sports Smart Training System for Android and iOS
- Pear Training-Intelligence (for iPhone)
- Pear Sports Mobile Intelligence Training System for iPhone Review
Under Armour Armour39 strap – ANT+
- phone compatibility – compatible with iPhones, iPods, and iPads (4 or later model)
- not compatible with Android phones
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: 400 hrs
- cost – $75-80
- nice feature – transmits your data in “real time” into MapMyFitness Apps, or into the Armour39 App, or to the Under Armour Watch
Jarv Premium Bluetooth 4.0 strap – Bluetooth 4.0 low energy with Bluetooth Smart technology
- phone compatibility – compatible with iPhones, iPods, and iPads (4 or later model)
- Works with most Android 4.3 or later devices
- Software compatibility – not compatible with Apple Healthkit, but compatible with over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: 400 hrs
- cost – $35 (big price drop recently)
- nice feature – comes with several free software apps – MapMyRun, RunMeter, EndoMondo, LogYourRun, and RunKeeper
- Plus, it is cheap!
Xyzer HRM strap – Bluetooth 4.0 low energy with Bluetooth Smart technology – one of those that are both iPhone and Android compatible
- phone compatibility – compatible with iPhones, iPods, and iPads (4 or later model)
- not compatible with Android phones
- software compatibility – not compatible with Apple Healthkit, but compatible with over 50 Smartphone apps
- onboard memory – no
- accelerometer/pedometer – no
- battery life: 400 hrs
- cost – $29-40 (big price drop recently, perhaps the cheapest belt sensor system)
- nice feature – compatible with either phone platform
Ear and Finger sensor systems
These systems are not as well-developed (none are wireless) or are not as “user friendly.” However, these have been around longer and the software that comes with them seems to be better than that for the chest straps.
HeartMath earlobe sensors – 6400 Inner Balance, EmWave Pro Model 6030 – HRV sensor that mounts on earlobe. A $299 stand-alone package with an earbud and hand-held monitor
The Journey to Wild Divine finger sensors (DeePak Chopra endorsed) – Biofeedback device that senses HRV from 3 finger sensors
Tink Wellness Sensor – this is a finger sensor that picks up HRV, respiratory rate, and pulse oximetry (blood oxygen saturation) – a combination not now available in chest or wrist sensor systems
Wrist-based HRV sensors
Including the “Breathing Coach” (W/Me) and the two 24/7 Automatic activity/sleep trackers (Reign and Basis Peak)
There is a growing interest in developing a HRV device that automatically tracks both activity and sleep with no straps, no wires, and no need to have a phone constantly linked. This currently means that the device must be wrist mounted and have an internal data storage feature that can be downloaded to a smart phone. If such a device is going to be created in the future, the future is already here!
There are at least three devices that fit into this category.
The first one mentioned here is not yet for sale – it is a proposed product created by a Kickstart, crowd-funded company. This device is not a 24/7 device, from what we know, The second and third ones mentioned here are both “24/7 activity/sleep trackers” that are waterproof and stylish. The Jaybird Reign is a wrist band, whereas the Basis Peak looks more like a watch.
The Jaybird Reign measures HRV, whereas the Basis Peak does not, however, there is hope that the new owner of Basis Peak (Intel) will add this feature.
W/Me – The Wrist band HRV monitor designed as a breathing coach
This is a Kickstarter company that has been funded with $140,000 of crowd sourcing money. They don’t yet have the wrist sensor in the market, but their prototypes and marketing information look great!
- Technology: a wristband style of wearable
- Claims to state, your agility score, and your autonomic system age
What it does for you: this is a breathing coach program
- See the Kickstarter writeup Finally, a wearable device that can improve your life. A HRV sensor sends your measurements via Bluetooth to your phone. The info is then downloaded into a spectrum analyzer, which then figures out your mental state and helps you as a breathing coach.
- compatibility: iOS with Bluetooth 4.0 (iPhone 4S or higher)
- battery: rechargeable via USB port. 7 day battery life
- cost: $55 for early access. $139 once it comes out
- features: no chest strap, ear lob device, or finger device needed
- what it tells you: It purports to calculate your mental state
- pros: very modern and stylish
- cons: Based on limited existing capitaliation, company must be in a very early stage. Unclear if the device delivers on its marketing promises. Unclear if it gives you any info on HRV power spectrum (i.e. numbers on LF, HF, etc.). Unclear if and when the product will actually hit the marketplace. “Tis many a slip twix the cup and the lip.”
Jaybird Reign – The Wrist band HRV monitor, Activity tracker, Sleep monitor, with unique feedback features for sleep decision making
- This device is from Austrailia and is already on the market that targets HRV for training recovery decisions (i.e. a rest coach) and for deciding how much sleep you need (i.e. a sleep coach)
- This is a stand-alone wrist band HRV monitor that has been designed primarily to help you determine when your body is fatigued and should rest – They call it the “Go-Score”, which is when you have fully recovered and are ready to do strenuous workouts again. It also tells you how much sleep you need and whether your sleep was fitful.
- compatibility: iOS with Bluetooth 4.0 (iPhone 4S or higher) now
- Android software will be available in 2015 for all Android 4.3 or higher devices
- battery life: Lithium batter that lasts 5 days
- water? lifetime waterproof warranty
- cost: $180-200
- features: no chest strap, waterproof, stylish magnetic pin-locking technology for strap
- more info here.
- Pros: The Jaybird Reign won the Outdoor Retailer 2014 “Gear of the Show” award
- It also won the “Best Digital Health and Fitness Product” award at CES 2014
- It also won SlashGear’s “Best Wearable Tech” award at CES2014
- Cons:, does not display HRV power spectrum, missing many practical features commonly available such as a display, alarms, “Can’t see trends over time. Doesn’t track heart rate. No calorie-logging system. Rudimentary mobile app. No desktop or Web app. No integration with third-party apps. No real display; LED indicators difficult to read. Difficult to fasten. Sensor pops out easily. So-so battery life.(ref)”
- online review: From PC magazine Rated only fair because of negatives just listed.
Basis Peak Ultimate Fitness & Sleep Tracker – The Wristwatch HR monitor (does not measure HRV at this time), Activity tracker, and Sleep monitor that also has a skin temperature sensor and a sweat sensor- Bluetooth 4.0
- This is a second-generation wristband devices made by a company called Basis that was recently cquired by Intel Corp,
- It is a specialized “smartwatch” designed as a “24-7 automatic fitness and sleep tracker”
- The sensors it embodies include a 24-7 continuous heart rate monitor, an accelerometer, a galvanic skin resistance sensor, and skin and ambient temperature sensors
- Since the Basis company is now owned by Intel, they may possibly update the device with software or hardwired versions that offer HRV
- It can measure stress and various stages of sleep using its accelerometer – measures REM sleep, light sleep, and deep slee
- The constitutional stress biomarkers proposed by Vince in the Wearables Part 2 blog entry were based directly on data from his Basis Peak
- compatibility: iOS with Bluetooth 4.0 (iPhone 4S or higher) now
- Android software for all Android 4.3 or higher devices
- battery: rechargeable battery with magnetic charging cradle, 4 day battery life (Vince gets only 3 days)
- screen: touch screen
- cost: $200
- water?: waterproof to 5 atmospheres
- features: no chest strap, waterproof, stylish magnetic pin-locking technology for strap
- more info: https://www.mybasis.com
- Pros: The Basis Peak watches have won several editor’s choice awards, including PC magazine
- A recent Basis Peak software upgrade allows the Peak to show smartwatch-type notifications originated from the companion smartphone
- It zeroes in on habits, rather than raw numbers.
- Excellent web interface
- Cons: It does not measure distance traveled, stairs climbed, breathing, oxygen saturation or many other biomarkers measurable using wearables.. It also does not now measure heart rate variability
- Online review of the Basis Peak
A comparison on the Jay Bird Reign and the Basis Peak can be found here, including “The top 20 reasons for the Basis Peak.”
Vince’s comments; See the Part 1 and Part 2 Wearables blog postings for my experience with the Basis Peak and what I believe are good constituitive stress biomarkers that can be derived from its measurements. I find these very exciting and believe that they could be practically superior to HRV in practice by virtue of their stability, reliability and ease of measurement. It looks like the Jaybird Reign may be a good competitor to the Peak, and currently possibly superior in reporting in some respects. As I point out in these blog entries my impression is that the Basis Peak offers an excellent hardware platform but that its software may not yet come close to providing the biomarker measurements that might be derived from its sensor outputs. In particular, I strongly suspect its second-generation heart rate measurement sensors could be used to create HRV measurements if the Basis company saw this as an objective. I mention also that a few competing products have just come on the market that are promoted as generating measurements similar to those obtainable from the Basis Peak. I am thinking particularly of the FitBit Charge HR, the Fitbit Surge and the Jawbone UP3. The above comments regarding the Peak may also apply to these products.
Using the iPhone or Android phone camera to measure HRV
An iPhone camera can be used as a photoplethysmography (PPG) device. This means it detects changes in blood volume during the cardiac cycle. Using the iPhone camera and the PPG technique, you can calculate HRV
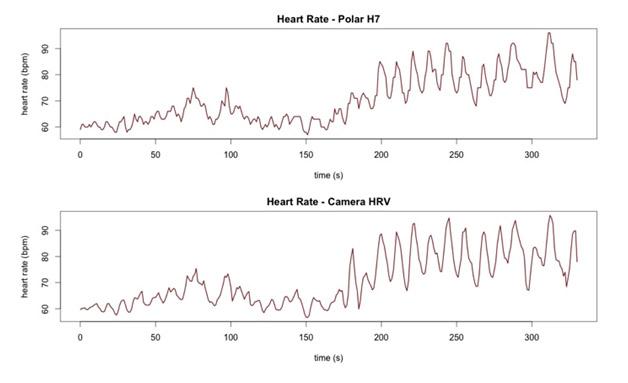
Here is an article on this: Heart rate variability using the phone’s camera.
The correlation between an iPhone camera based HRV monitor and a Polar H7 is very close (0.97). Here is the diagram to show this:
- This approachbasically eliminates the need for any heart rate sensor hardware
- You just put your finger up to the iPhone camera and it senses your HR and calculates your HRV
- The measurements may not be that accurate, but clearly the simplest, easiest, and cheapest way to measure HRV
- Here are some places you can download an app to do this
- Camera Heart Rate Variability – this one is $3.99
- Stress Check Pro by Azumio – this is a $1.99 app
- Instant Heart Rate by Azumio – this one is for free
Azumio Instant Heart Rate Pro is also available for use with selected Android phones on Google Play
More Sophisticated Sensors
These devices are much more sophisticated than the above devices. They have more sophisticated capabilities, such as measuring respiration (breathing), skin temperature, and, reportedly, HRV.
Zephyr BioModule BH3 removable sensor system - This is a strapBluetooth 4.0 low energy strap that is much more sophisticated than the straps above, for Android devices
- The sensor is removable and can be mounted on a strap, a compression shirt with a pocket for the sensor, or a loose fit shirt
- The sensor has a respiration sensor, a 3-axis accelerometer, and HR/HRV sensor
- Sold as a performance monitoring device for serious athletes
Vital Connect’s Health Patch – This is a strapless Bluetooth 4.0 low energy patch
- It can measure HR, HRV, respiratory rate, skin temperature, body posture, steps, sleep staging
- However, they do not have software out for this yet, so you have to use Sweetbeat’s software, which is what I am using now with the Polar H7 chest strap.
- See the Sweetwater HRV site and the Vital Connect store site
Actiheart system by Camntech – This is a strapless (or strap) system that can be stuck on the skin with standard ECG electrode patches
- One electrode goes over the sternum and the other electrode is 10 cm away over the “V5″ or “V6″ position of an ECG (this gives better data)
- It can also be used with a strap and can record heart rate and activity for up to 21 days.
- Unfortunately, it is not a Bluetooth 4.0 low energy system
- It can also record “Inter-beat-Interval”, which is used to calculate HRV. Here is some more info on this:
HRV Software
Software for Bluetooth HRV devices -
BioForce – This is a highly rated HRV app for Blue tooth HR sensor devices – Android
Senseview – This is a free software that is designed for the Android 4.0 dual core processor
Sweetbeats – This one is $4.99. It is the one I am using. Currently Apple iOS only but an Android version has gone into alpha testing. My rating: not that good
ithlete - This one is expensive. Android and Apple iOS
Heart Rate Variability Logger – this one is free – Apple iOS
SelfLoops HRV – this one is free – Apple iOS
HRV tracker – this one is also free – Apple iOS
Heart Rate + Cardiorespiratory Coherence – this one is $4.99. My rating: from what I can see, this may be the best one out there
MyCalmBeat – this one is free
Buddha Mind – this one is $2.99 – Apple iOS. My Rating: I like this one, from what I have read. There are 120,000 downloads, but 58% say there are problems with it
Expert by CardioMood – $1.69 – Android only
May be the best non-professional Android HRV app. Offers much information including spectral power breakdowns. This is one of the HRV apps that Vince has been using with his Polar H7 belt. Vince likes the wealth of readouts, but has been unable to generate measurements with consistency and reliability. Vince relates that on five occasions he has made three measurements in a row while in the same sitting position without touching the belt or changing position or modifying anything else. The way Vince puts it is that one of the back-to-back measurements shows a 50% or more high frequency HRV power measurement, stress of 60 and the profile of a healthy athlete; another of the measurements shows 75% of the HRV power is in the low and ultralow frequency spectrum and the stress index is over 300. And the third measurement is so screwy that if Vince were to take it seriously, he would have his wife call an ambulance to rush him to an emergency room. These variations seem to appear in most multi-reading session Vince has done with the software. So something is seriously wrong in the software, the belt, or in what Vince has been doing.
Elite HRV – free – Android
Has a neat and simple dashboard and rated with 4 stars. Produces only two numbers: heart rate and a HRV reading. Company is in development, Does not appear to offer a web data interface yet. This is another HRV app that Vince has been using. Produces back-to-back HRV measurement that are not always consistent. Three readings taken in a row for Vince may show stress indices of 36, 76 and 58.
************************
Vince and I expect to report on our personal HRV measuring experiences and measurements in a subsequent blog entry. There we expect to compare the ease and efficacy of measuring HRV as against Vince’s stress biomarkers MRHR (morning resting heart rate just before waking up) and ERHR-MRHR (drop in sleeping resting heart rate overnight).
View the full article at Anti-Aging Firewalls