Featured





 3 votes
3 votes
Geroprotector review: Rapamycin
Posted by
s123
,
18 July 2017
·
18,214 views
rapamycin
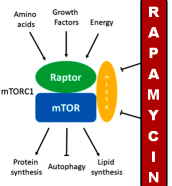
Rapamycin and other mTOR inhibitors as geroprotectors
This article is solely for information purposes, not a substitute for professional medical or dietary advice. The provisos of the LongeCity user agreement apply.
| What: A drug to prevent rejection of transplanted kidneys. Also used in cancer therapy. Various related compounds (‘-olimus’ /’rapalogs’) Lifespan: Increase in yeast, roundworm, fruit flies, and rodents, Cancer: Potentially reduced risk in nonmelanoma skin cancers. Maybe increased risk for prostate cancers. Heart disease: Prevention of atherosclerosis, reduction in stroke severity, possible improvement in heart function. Alzheimer’s disease: Protective in a mouse model of Alzheimer’s disease. Mechanism: Inhibition of mTORC1 leading to upregulation of authophagy and downregulation of protein and lipid synthesis and metabolism. Attenuation of cellular senescence and senescence-associated secretory phenotype. May prevent progerin accumulation, age related changes in the epigenome and improve hematopoietic stem cell function. Side effects: Main side effects are insulin resistance, glucose intolerance and hyperlipidemia, decreased immune function, delayed wound healing and mouth ulcers. However, it has been argued that the insulin resistance, glucose intolerance and hyperlipidemia may be benign. Improved immune response to vaccination in elderly humans but worsens infection risk and outcome of acute infections. |
Introduction
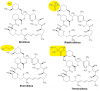
Rapamycin, also known as Sirolimus, is an immunomodulatory compound used to prevent rejection of transplanted kidneys. It was first isolated in 1972 by Surendra Nath Sehgal and colleagues from a strain of the bacterium Streptomyces hygroscopicus isolated from soil samples from Easter Island. The native name for Easter Island is Rapa Nui hence the name rapamycin. It was originally developed as an antifungal drug. When the immunosuppressive effects were discovered, it opened up a new line of research in preventing the rejection of transplanted organs (Arriola Apelo and Lamming, 2016). Since then, many derivatives of rapamycin have been developed including temsirolimus, everolimus, ridaforolimus, zotarolimus, umirolimus and others, collectively known as rapalogs (Figure 1).
 (click to enlarge) Figure 1 Some examples of rapalogs. The difference is highlighted in yellow.
(click to enlarge) Figure 1 Some examples of rapalogs. The difference is highlighted in yellow.Dose and dosage schedule
We still have not discovered the optimal rapamycin dose for lifespan extension. For example, in a dose-response study (4.7, 14 or 42 ppm) it was found that the highest dose resulted in the largest lifespan extension (Miller et al., 2014). So, it may be that many published studies have used rapamycin well below the optimal dose (Kaeberlein, 2014). One study that found that rapamycin improved survival in a mouse model of Leigh syndrome (see below) used a dose of rapamycin leading to a blood concentration of rapamycin at least 20-fold higher than what has been observed in ‘normal aging’ studies with rapamycin (Johnson et al., 2013).
In contrast , it has been found that giving rapamycin less frequently (for example once a week rather than every day) prevented some of the side effects. For example, while daily rapamycin treatment results in significant higher blood sugar levels in an oral glucose tolerance test, mice treated with rapamycin once a week had a similar blood glucose profile as those in the control group. Once a week rapamycin treatment also had a significant reduced impact on immune function (as measured by the abundance of various immune cells) compared to daily rapamycin. However, immune markers at once a week rapamycin treatment were still different from controls (Arriola Apelo et al., 2016).
The rapalogs, everolimus and temsirolimus, have a reduced impact on glucose tolerance and immune function compared to rapamycin (Arriola Apelo et al., 2016). The search for rapalogs that have an even better safety profile is still ongoing (see below).
In animal studies, rapamycin is typically administered in an micro-encapsulated formulation (EUDRAGIT®) that is mixed into the diet. Encapsulation prevents the degradation of the rapamycin by the acidic conditions of the stomach (WO 2015103447 A1). Furthermore, it is believed that the microencapsulation of rapamycin could help to prevent mouth ulcers, a common side effect observed with use of non-encapsulated rapamycin (Tardif et al., 2015).
Lifespan extension
Rapamycin has been found to increase lifespan in yeasts (Powers et al., 2006; Medvedik et al., 2007; Rallis et al., 2013), worms (Robida-Stubbs et al., 2012), and fruit flies (Bjedov et al., 2010; Moskalev and Shaposhnikov, 2010). Furthermore, the rapalog everolimus extends lifespan in fruit flies (Spindler et al., 2012).
 (click to enlarge) Figure 2 Lifespan extension by rapamycin in non-transgenic rodents.
(click to enlarge) Figure 2 Lifespan extension by rapamycin in non-transgenic rodents.In 2009 a landmark paper was published in Nature. In this paper the results from the Interventions Testing Program (ITP) with rapamycin in middle-aged mice were reported. Rapamycin significantly extended the mean and maximal lifespan in both male and female mice. Since multiple studies have confirmed that rapamycin extends lifespan in rodents (see figure 2).
Not all studies have published data that allowed me to include them in Figure 2. Chen et al. (2009) fed rapamycin every other day to old (22-24 month) mice for 6 weeks and observed a significant reduction in mortality rate. Similarly, Neff et al. (2013) observed a significant lifespan extension upon rapamycin treatment in male C57BL/6J Rj mice.
Neff et al. (2013) measured a wide array of age-related phenotypes and found that rapamycin improved some but not others (see table 1). From this the authors concluded that rapamycin has little effect on aging. However can we really expect that a single life extending drug would beneficially impact all age-related phenotypes?
Rapamycin has been tested in various transgenic mouse models (at least 16 models so far). Lifespan was extended in most transgenic models except in a mouse model of type two diabetes and in two models of amyotrophic lateral sclerosis (ALS) (Richardson et al., 2015; Arriola Apelo and Lamming, 2016). Most impressive are the results from two studies with mice harboring mutations in APC. APC mutations lead to colorectal cancer. In these mice lifespan was extended by 140-220% (Fujishita et al., 2008) and 280-440% (Hasty et al., 2014). Of course, mice harboring mutations in APC have a strongly reduced lifespan compared to wild type mice. Interestingly, Hasty et al. (2014) observed that the APC mutant mice fed rapamycin at the highest dose (42 ppm) lived longer than the typical median lifespan reported in the literature for wild type mice! Rapamycin also increases lifespan of mice who lack one or both copies of the p53 tumor suppressor gene (Comas et al., 2012; Komarova et al., 2012).
Combination with metformin
The combination of metformin with rapamycin in the Interventions Testing Program (ITP) lead to a larger lifespan extension than either drug in isolation (Strong et al., 2016). Although, to compare rapamycin + metformin with rapamycin alone the data from the rapamycin alone group was derived from previous ITP studies. The fact that the two groups were not studied simultaneously introduces the risk that a change in some unknown variable could make this comparison invalid. In worms the combination of rapamycin with metformin each at optimal dose does not lead to any additional lifespan extension compared to either alone. However when used at suboptimal doses then the combination lead to a bigger increase in maximal but not mean lifespan compared to either alone (Dessale et al., 2017). Metformin is also a well known potential life extension drug (http://www.longecity.org/forum/blog/201/entry-3593-geroprotector-review-metformin/) and inhibition of mTORC1 (the target of rapamycin, see below) is one of the mechanisms that has been proposed to explain its effects.
Human data are scarce but one study of kidney transplant patients found that mortality was increased in those who had received a transplant from a deceased donor while mortality was unchanged in those receiving a transplant from a living donor (Knoll et al., 2014). Another meta-analysis also observed no effect from rapamycin on mortality in kidney transplant patients (Liu et al., 2017). However, these data should be interpreted with great care as mortality in the years after a transplant is more dependent on factors such as rejection of the transplanted organ than from age-related diseases.
Effect on aging phenotypes and diseases
Rapamycin treatment in mice improved some but not all phenotypes of aging (Wilkinson et al., 2012; Neff et al., 2013).
 (click to enlarge) Table 1 Age-related phenotypes in rapamycin treated mice. Data from (Wilkinson et al., 2012; Neff et al., 2013; Bitto et al., 2016).
(click to enlarge) Table 1 Age-related phenotypes in rapamycin treated mice. Data from (Wilkinson et al., 2012; Neff et al., 2013; Bitto et al., 2016).Rapalogs have been found to be protective against atherosclerosis in mouse models (Pakala et al., 2005; Mueller et al., 2008; Ma et al., 2013). Furthermore, rapamycin protected animals against experimentally-induced stroke. Rapamycin treated animals had a smaller infarct area, were protected from ischemia-induced cell death, and motoric impairment was attenuated (Sheng et al., 2010; Chauhan et al., 2011; Yang et al., 2015). Rapamycin attenuates load-induced cardiac hypertrophy in mice (Shioi et al., 2003). Rapamycin did not improve heart function as measured by echocardiography in mice (Neff et al., 2013). In contrast, ten weeks of rapamycin treatment was found to improve heart function as measured by echocardiography in companion dogs (Urfer et al., 2017). However, this was a small proof-of-principle trial with just 24 dogs. The researchers now prepare to scale up this trial and possibly run it in multiple centers around the US.
Rapamycin was found to improve memory in two mouse models of Alzheimer’s disease (Caccamo et al., 2010; Spilman et al., 2010; Majumder et al., 2011; Lin et al., 2013). Rapalog treatment may improve several of the key pathophysiological factors observed in Alzheimer’s disease.
-- rapalogs reduce tau tangles and tau hyperphosphorylation (Ozcelik et al., 2013; Jiang et al., 2014a).
-- rapalogs promote the autophagic clearance of amyloid-beta leading to lower amyloid-beta levels in the brain (Spilman et al., 2010; Jiang et al., 2014b). However, one contrasting study observed increased amyloid-beta levels in the-- brain of a mouse model of Alzheimer’s disease (Zhang et al., 2010).
-- rapalogs reduce neuroinflammation (Wang et al., 2017a).
-- rapamycin restores cerebral blood flow and vascular function in a mice model of Alzheimer’s disease (Lin et al., 2013).
Rapamycin has also been found to be neuroprotective in mice models of Parkinson’s disease (Malagelada et al., 2010; Jiang et al., 2013).
We have already discussed the potential effect of rapamycin on colon cancer (see APC mice above). Now what about other cancers? Rapamycin has been found to lower spontaneous cancer incidence in mice (Neff et al., 2013). Everolimus and temsirolimus have received FDA and EMA approval for the use as monotherapy against various cancers (Eiden et al., 2016). In a meta-analysis of human randomized, controlled clinical trials it was found that rapamycin use in people who had undergone a kidney transplant was associated with a reduced risk of cancer. However, this decrease was caused by a strong reduction in the incidence of nonmelanoma skin cancer. When this type of cancer was excluded then rapamycin no longer influenced cancer risk except for a lower risk for kidney cancer and a higher risk for prostate cancer (Yanik et al., 2015). In another meta-analysis of liver transplant recipients rapamycin was found to lower both nonmelanoma skin cancer as well as other cancers (Knoll et al., 2014).
Rapalogs are known immunosuppressive drugs and hence it would be expected that their use was associated with worse outcomes during infections. Unexpectedly, rapalogs improves the response to vaccination in mice (Jagannath et al., 2009; Jagannath and Bakhru, 2012; Keating et al., 2013) and in elderly humans (Mannick et al., 2014). Rapamycin is however deleterious during acute infections (Goldberg et al., 2014).
Other diseases
Rapamycin is beneficial in mouse models of several autoimmune diseases including (Donia et al., 2009; Esposito et al., 2010; Prevel et al., 2013).
In mice it was also found that rapamycin worsened cataract severity (Wilkinson et al., 2012).
Daily injection of rapamycin more than doubled lifespan in a mouse model of Leigh syndrome. Furthermore disease symptoms were absent in nearly half of the mice injected with rapamycin (Johnson et al., 2013).
Rapamycin is FDA approved for the treatment of the rare, progressive and systemic disease lymphangioleiomyomatosis that results in cystic destruction of the lung(link).
Mechanism of action
Rapamycin works by inhibiting the mTORC1 complex. Consistent with the fact that rapamycin extends lifespan is the observation that mutations that downregulate mTORC1 signaling extend lifespan in yeast, worms, fruit flies, and mice (Wu et al., 2013; Kaeberlein, 2014). mTORC1 is a protein complex composed of mTOR (mechanistic target of rapamycin) with several other proteins that integrates a large set up input signals such as energy status, amino acid levels, growth factors, stress, oxygen availability and when activated stimulates cell growth and proliferation by promoting protein synthesis, lipid synthesis, and metabolism while reducing autophagy (Laplante and Sabatini, 2013; Saxton and Sabatini, 2017).
The contribution of the various downstream effectors of mTORC1 on lifespan is not fully understood so far. Reducing protein synthesis by knocking out the downstream effector of mTORC1, S6K, increases lifespan in worms (McQuary et al., 2016) and female mice (Selman et al., 2009). It is also known that autophagy induction is necessary for lifespan extension by various interventions such as calorie restriction (Madeo et al., 2015). Rapamycin fails to extend lifespan in fruit flies in which the downstream targets of mTORC1 (S6K, 4E-BP and autophagy) have been made to be insensitive to mTORC1 signalling (Bjedov et al., 2010). Hence, both protein synthesis and autophagy may be necessary for rapamycin’s life-extending effects.
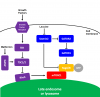 (click to enlarge) Figure 3 mTORC1 and mTORC2 signaling. The blunted arrows indicate inhibition. For simplicity: both complexes are drawn as monomers, in reality they form homodimers; not all upstream and downstream effectors have been listed.
(click to enlarge) Figure 3 mTORC1 and mTORC2 signaling. The blunted arrows indicate inhibition. For simplicity: both complexes are drawn as monomers, in reality they form homodimers; not all upstream and downstream effectors have been listed.mTORC1 is strongly activated by the branched-chain amino acids (leucine, isoleucine, and valine). Leucine directly inhibits Sestrin2 thereby abrogating its inhibitory action on GATOR2 leading to the activation of mTORC1 (see figure 4). The amino acid sensors that signal for mTORC1 activation have been reviewed by Goberdhan et al. (2016). New drugs to inhibit mTORC1 have been developed that make use of this upstream amino acid sensing mechanism (see below).
Rapamycin suppresses cell senescence (Demidenko et al., 2009; Pospelova et al., 2012) and reduces the senescence-associated secretory phenotype in cells that are already senescent (Laberge et al., 2015; Wang et al., 2017b). Furthermore, pan-mTOR inhibitors (compounds that block both mTORC1 and mTORC2) have been shown to prevent cell senescence (Leontieva and Blagosklonny, 2016). Rapamycin treatment reversed the cellular phenotype of Hutchinson-Gilford progeria syndrome in cell culture. Furthermore, it prevented the accelerated onset of cell senescence and increased the degradation of progerin in cells from Hutchinson-Gilford progeria syndrome patients (Cao et al., 2011). Progerin accumulation, the molecular defect responsible for Hutchinson-Gilford progeria syndrome, has also been observed during ‘normal’ aging although at much lower amount (Graziotto et al., 2012). It has also been shown that rapamycin attenuates age-related changes in DNA methylation (Cole et al., 2017). Finally, rapamycin treatment restores hematopoietic stem cell function in old mice (Chen et al., 2009).
However chronic use of rapamycin may also lead to an inhibition of mTORC2 in certain tissues probably as a result of an inability of rapamycin-bound mTOR to be incorporated into new mTORC2 complexes (Schreiber et al., 2015). The inhibition of mTORC2 by chronic rapamycin treatment is a possible explanation for the glucose intolerance observed in several studies. mTORC2 is needed for full activation of Akt which in turn is needed for glucose uptake in the cell. Hence when mTORC2 is inhibited glucose uptake is also reduced.
Side effects of rapamycin
In several but not all strains of mice rapamycin treatment causes glucose intolerance and insulin resistance (Liu et al., 2014; Kennedy and Lamming, 2016). Human data similarly indicate that rapamycin causes glucose intolerance but we have to be cautious in interpretation of the human data as all of it comes from seriously ill people who are often treated with other drugs known to induce metabolic disturbances (e.g. steroids to prevent organ rejection). Surprisingly enough in a mouse study it was found that short term (2 or 6 weeks) of rapamycin had negative effects on insulin resistance and blood triglyceride levels while these spontaneously reversed after 20 weeks treatment. Furthermore, after 20 weeks of rapamycin treatment some parameters (such as insulin resistance) were even better than the control group (Fang et al., 2013). Other studies find that the glucose intolerance caused by rapamycin is quickly reversible upon cessation of treatment, although not strong enough to return to baseline levels (Schindler et al., 2014). Rapamycin improved insulin sensitivity in three out of five mouse models of type 2 diabetes (Reifsnyder et al., 2016). Fourteen months of rapamycin treatment did not cause glucose intolerance in marmoset monkeys (Ross et al., 2015). All these contradictory data make it very hard to come to any solid conclusions concerning rapamycin’s effect on glucose intolerance and insulin sensitivity. Also, not everyone agrees that the glucose intolerance and insulin resistance caused by rapamycin is harmful. Mikhail Blagosklonny argues that rapamycin mimics “starvation diabetes” (Blagosklonny, 2011; 2012; 2014).
As mentioned before, mouth ulcers are a common side effect of rapamycin treatment but these might be preventable by using an enteric-coated formulation such as EUDRAGIT® (Tardif et al., 2015). Rapamycin has also been found to delay wound healing (Kahn et al., 2005; Ekici et al., 2007; Willems et al., 2010).
Newer mTOR inhibitors
Rapamycin does not directly bind to the mTOR protein rather it first binds to a protein from the FKBP family and then the rapamycin-FKBP complex binds to mTORC1 leading to its inhibition (see figure 3). Many of the 14 known family members can form complexes with rapamycin and inhibit mTOR activity (März et al., 2013). Different cell types express different FKBP family members (Baughman et al., 1997; Rulten et al., 2006). Rapamycin-bound FKBP51 inhibits only mTORC1 and not mTORC2. In contrast rapamycin-bound FKBP12 can inhibit mTORC1 but after chronic exposure to rapamycin it also inhibits mTORC2 (see figure 3).
Delos Pharmaceuticals and Buck Institute for Research on Aging have recently filed a patent on new rapalogs that are more selective towards mTORC1 (WO 2017040341 A1).
Rather than trying to block mTOR directly Navitor Pharmaceuticals is testing an alternative approach in which a drug stimulates the inhibitory effect of Sestrin2 on GATOR2 leading ultimately to an inhibition of mTORC1. Navitor Pharmaceuticals has recently filed a patent on Sestrin2/GATOR2 modulators (WO 2017044720 A1).
However, not everyone agrees with this reasoning. Mikhail Blagosklonny argues that the hyperglycemia and hyperlipidemia caused by rapamycin treatment are benign. He has in fact taken the complete opposite strategy and is looking at pan-mTOR inhibitors that are designed to block both mTORC1 and mTORC2. Using a reversible model of senescence he was able to demonstrate that pan-mTOR inhibitors prevent geroconversion (Leontieva and Blagosklonny, 2016).
Rapamycin is currently being tested in marmoset monkeys. Marmoset monkeys have a shorter lifespan than other monkey species. Data from these experiments have confirmed that rapamycin reduces mTORC1 activity (Tardif et al., 2015) and have not observed any glucose intolerance or increase in blood lipids after 14 month treatment (Ross et al., 2015). The NIA has awarded the researchers a $2.7 million grant to investigate the effect of rapamycin on the lifespan of these monkeys (https://www.sciencedaily.com/releases/2016/02/160209105352.htm).
Conclusions
Like many potential life extension promoters, rapamycin comes from a different field of clinical activity and testing it in ‘normal aging’ is challenging and likely to reveal new benefits and long term toxicities. While there is hope for rapamycin as a broad-spectrum drug, it is unlikely to be is the groundbreaking anti-aging intervention in humans that it was sometimes made out to be, but a better understanding of the mTOR pathway that rapamycin and its derivatives inhibit is likely to play an important role in developing effective life extension strategies.
References
- Anisimov VN et al. (2010). Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol 176(5): 2092-2097.
- Anisimov VN et al. (2011). Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 10(24): 4230-4236.
- Arriola Apelo SI, Lamming DW (2016). Rapamycin: An inhibiTOR of aging emerges from the soil of Easter Island. J Gerontol A Biol Sci Med Sci 71(7): 841-849.
- Arriola Apelo SI et al. (2016). Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell 15: 28-38.
- Bitto A et al. (2016). Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5: e16351.
- Bjedov I et al. (2010). Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 11: 35-46.
- Blagosklonny M (2011). Rapamycin-induced glucose intolerance: hunger or starvation diabetes. Cell Cycle 10: 4217-4224.
- Blagosklonny M (2012). Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 4(5): 350-358.
- Blagosklonny M (2014). Koschei the immortal and anti-aging drugs. Cell Death Dis 5: e1552.
- Baughman G et al. (1997). Tissue distribution and abundance of human FKBP51, and FK506-binding protein that can mediate calcineurin inhibition. Biochem Biophys Res Commun 232: 437-443.
- Caccamo A et al. (2010). Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and tau: effects on cognitive impairments. J Biol Chem 285(17): 13107-13120.
- Chauhan A et al. (2011). Rapamycin protects against middle cerebral artery occlusion induced focal cerebral ischemia in rats. Behav Brain Res 225(2): 603-609.
- Chen C et al. (2009). mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal 2(98): ra75.
- Cole JJ et al. (2017). Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Genome Biol 18: 58.
- Comas M et al. (2012). New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging 4: 715-722.
- Demidenko ZN et al. (2009). Rapamycin decelerates cellular senescence. Cell Cycle 8(12): 1888-1895.
- Dessale T et al. (2017). Slowing ageing using drug synergy in C. elegans. bioRxiv: 153205. http://biorxiv.org/content/early/2017/06/23/153205
- Donia M et al. (2009). Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing experimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4+CD25+Foxp3+ regulatory T cells. J Autoimmun 33(2): 135-140.
- Eiden AM et al. (2016). Molecular Pathways: Increased Susceptibility to Infection Is a Complication of mTOR Inhibitor Use in Cancer Therapy. Clin Cancer Res 22(2): 277-283.
- Ekici Y et al. (2007). Effect of rapamycin on wound healing: an experimental study. Transplant Proc 39(4): 1201-1203.
- Esposito M et al. (2010). Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. J Neuroimmunol 220(1-2): 52-63.
- Fang Y et al. (2013). Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab 17: 456-462.
- Fok WC et al. (2014). Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One 9: e83988.
- Fujishita T et al. (2008). Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci USA 105: 13544-13549.
- Goberdhan DC et al. (2016). Amino acid sensing by mTORC1: intracellular transporters mark the spot. Cell Metab 23: 550-589.
- Goldberg EL et al. (2014). Immune memory-boosting dose of rapamycin impairs macrophage vesicle acidification and curtails glycolysis in effector CD8 cells, impairing defense against acute infections. J Immunol 193(2): 757-763.
- Graziotto JJ et al. (2012). Rapamycin activates autophagy in Hutchinson-Gilford progeria syndrome: Implications for normal aging and age-dependent neurodegenerative disorders. Autophagy 8(1): 147-151.
- Harrison DE et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460(7253): 392-395.
- Hasty P et al. (2014). eRapa restores a normal life span in a FAP mouse model. Cancer Prev Res (Phila) 7: 169-178.
- Jagannath C et al. (2009). Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med 15(3): 267-276.
- Jagannath C, Bakhru P (2012). Rapamycin-induced enhancement of vaccine efficacy in mice. Methods Mol Biol 821: 295-303.
- Jiang J et al. (2013). Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson's disease. Int J Mol Med 31(4): 825-832.
- Jiang T et al. (2014a). Temsirolimus attenuates tauopathy in vitro and in vivo by targeting tau hyperphosphorylation and autophagic clearance. Neuropharmacology 85: 121-130.
- Jiang T et al. (2014b). Temsirolimus promotes autophagic clearance of amyloid-β and provides protective effects in cellular and animal models of Alzheimer's disease. Pharmacol Res 81: 54-63.
- Johnson SC et al. (2013). mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342: 1524-1528.
- Kaeberlein M (2014). Rapamycin and ageing: When, for how long, and how much? J Genet Genomics 41(9): 459-463.
- Kahn D et al. (2005). The effect of rapamycin on the healing of the ureteric anastomosis and wound healing. Transplant Proc 37(2): 830-831.
- Keating R et al. (2013). The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol 14: 1266-1276.
- Kennedy BK, Lamming DW (2016). The mechanistic target of rapamycin: The grand conducTOR of metabolism and aging. Cell Metab 23: 990-1003.
- Knoll GA et al. (2014). Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 349: g6679.
- Komarova EA et al. (2012). Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging 4: 709-714.
- Laberge RM et al. (2015). MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17: 1049-1061.
- Laplante M, Sabatini DM (2013). Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126(Pt 8): 1713-1719.
- Lelegren M et al. (2016). Pharmaceutical inhibition of mTOR in the common marmoset: effect of rapamycin on regulators of proteostasis in a non-human primate. Pathobiol Aging Age Relat Dis 6: 31793.
- Leontieva OV, Blagosklonny MV (2016). Gerosuppression by pan-mTOR inhibitors. Aging (Albany NY) 8(12): 3535-3549.
- Lin AL et al. (2013). Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab 33:1412-1421.
- Liu Y et al. (2014). Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging (Albany NY) 6(9): 742-754.
- Liu J et al. (2017). Efficacy and Safety of Everolimus for Maintenance Immunosuppression of Kidney Transplantation: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 12(1): e0170246.
- Ma KL et al. (2013). Increased mTORC1 activity contributes to atherosclerosis in apolipoprotein E knockout mice and in vascular smooth muscle cells. Int J Cardiol 168(6): 5450-5453.
- Madeo F et al. (2015). Essential role for autophagy in life span extension. J Clin Invest 125(1): 85-93.
- Majumder S et al. (2011). Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One 6: e25416.
- Malagelada C et al. (2010). Rapamycin Protects Against Neuron Death In in vitro and in vivo Models of Parkinson's Disease. J Neurosci 30(3): 1166-1175.
- Mannick JB et al. (2014). mTOR inhibition improves immune function in the elderly. Sci Trans Med 6(268): 268ra179.
- März AM et al. (2013). Large FK506-binding proteins shape the pharmacology of rapamycin. Mol Cell Biol 33(7): 1357-1367.
- McQuary PR et al. (2016). C. elegans S6K Mutants Require a Creatine Kinase-Like Effector for Lifespan Extension. Cell Rep 14(9): 2059-2067.
- Medvedik O et al. (2007). MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol 5: e261.
- Miller RA et al. (2011). Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66: 191-201.
- Miller RA et al. (2014). Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13: 468-477.
- Moskalev AA, Shaposhnikov MV (2010). Pharmacological inhibition of phosphoinositide 3 and TOR kinases improves survival of Drosophila melanogaster. Rejuvenation Res 13(2-3): 246-247.
- Mueller MA et al. (2008). Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR-/- mice despite severe hypercholesterolemia. Atherosclerosis 198: 39-48.
- Neff F et al. (2013). Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest 123(8): 3272-3291.
- Ozcelik S et al. (2013). Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS One 8(5): e62459.
- Pakala R et al. (2005). Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis. J Cardiovasc Pharmacol 46: 481-486.
- Pospelova TV et al. (2012). Suppression of replicative senescence by rapamycin in rodent embryonic cells. Cell Cycle 11(12): 2402-2407.
- Prevel N et al. (2013). Beneficial role of rapamycin in experimental autoimmune myositis. PLoS One 8(11): e74450.
- Rallis C et al. (2013). TORC1 signaling inhibition by rapamycin and caffeine affect lifespan, global gene expression, and cell proliferation of fission yeast. Aging Cell 12(4): 563-573.
- Reifsnyder PC et al. (2016). Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY) 8(11): 3120-3130.
- Richardson A et al. (2015). How longevity research can lead to therapies for Alzheimer’s disease: The rapamycin story. Exp Gerontol 68: 51-58.
- Powers 3rd RW et al. (2006). Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev 20: 174-184.
- Robida-Stubbs S et al. (2012). TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 15: 713-724.
- Ross C et al. (2015). Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus). Aging (Albany NY) 7(11): 964-973.
- Rulten SL et al. (2006). The human FK506-binding proteins: characterization of human FKBP19. Mamm Genome 17(4): 322-331.
- Santini E et al. (2009). Inhibition of mTOR Signaling in Parkinson’s Disease Prevents l-DOPA–Induced Dyskinesia. Sci Signal 2(80): ra36.
- Saxton RA, Sabatini DM (2017). mTOR signaling in growth, metabolism, and disease. Cell 168: 960-976.
- Schindler CE et al. (2014). Chronic rapamycin treatment causes diabetes in male mice. Am J Physiol Regul Integr Comp Physiol 307(4): R434-R443.
- Schreiber et al. (2015). Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell 14(2): 265-273.
- Selman C et al. (2009). Ribosomal protein S6 kinase 1 signaling regulates mammalian lifespan. Science 326(5949): 140-144.
- Sheng R et al. (2010). Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy 6(4): 482-494.
- Shioi T et al. (2003). Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 107(12): 1664-1670.
- Spilman P et al. (2010). Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5(4): e9979.
- Spindler SR et al. (2012). Novel protein kinase signaling systems regulating lifespan identified by small molecule library screening using Drosophila. PLoS ONE 7(2): e29782.
- Strong R et al. (2016). Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α‐glucosidase inhibitor or a Nrf2‐inducer. Aging Cell 15: 872-884.
- Tardif S et al. (2015). Testing Efficacy of Administration of the Antiaging Drug Rapamycin in a Nonhuman Primate, the Common Marmoset. J Gerontol A Biol Sci Med Sci 70(5): 577-588.
- Urfer SR et al. (2017). A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. GeroScience 39: 117-127.
- Yang X et al. (2015). Inhibition of mTOR Pathway by Rapamycin Reduces Brain Damage in Rats Subjected to Transient Forebrain Ischemia. Int J Biol Sci 11(12): 1424-1435.
- Yanik EL et al. (2015). Sirolimus effects on cancer incidence after kidney transplantation: a meta-analysis. Cancer Med 4(9): 1448-1459.
- Wang AC et al. (2017a). Rapamycin as a Novel Therapeutic for Alzheimer’s Disease: Prevention Assessed Through Neuroimaging. FASEB J 31(Suppl. 1): 814.6.
- Wang R et al. (2017b). Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 16(3): 564-574.
- Wilkinson JE et al. (2012). Rapamycin slows aging in mice. Aging Cell 11(4): 675-682.
- Willems MC et al. (2010). Persistent effects of everolimus on strength of experimental wounds in intestine and fascia. Wound Repair Regen 18(1): 98-104.
- Wu JJ et al. (2013). Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep 4(5): 913-920.
- Zhang S et al. (2010). Rapamycin promotes beta-amyloid production via ADAM-10 inhibition. Biochem Biophys Res Commun 398: 337-341.
- Zhang Y et al. (2014). Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci 69: 119-130.



















































