All:
A followup to the earlier Mannick et al open-label pilot study with everolimus, which only showed improved flu vaccine response:
TORC1 inhibition enhances immune function and reduces infections in the elderly
By Joan B. Mannick, Melody Morris, Hans-Ulrich P. Hockey, Guglielmo Roma, Martin Beibel, Kenneth Kulmatycki, Mollie Watkins, Tea Shavlakadze, Weihua Zhou, Dean Quinn, David J. Glass, Lloyd B. Klickstein
Science Translational Medicine
11 Jul 2018: Vol. 10, Issue 449, eaaq1564
DOI: 10.1126/scitranslmed.aaq1564
The objective of this phase 2a randomized, placebo-controlled clinical trial was to determine whether low-dose mTOR inhibitor therapy enhanced immune function and decreased infection rates in 264 elderly subjects given the study drugs for 6 weeks.
A low-dose combination of a catalytic (BEZ235 [=RTB101]) plus an allosteric (RAD001 [=everolimus]) mTOR inhibitor that selectively inhibits target of rapamycin complex 1 (TORC1) downstream of mTOR was safe and was associated with a significant (P = 0.001) decrease in the rate of infections reported by elderly subjects for a year after study drug initiation. In addition, we observed an up-regulation of antiviral gene expression and an improvement in the response to influenza vaccination in this treatment group.
[resTORbio Press Release]
In the RTB101 monotherapy and RTB101+everolimus combination treatment arms, statistically significant and clinically meaningful reductions in the annual rate of infections of 33% (p=0.008) and 38% (p=0.001), respectively, compared to placebo, were observed. In addition, both RTB101 monotherapy and the RTB101+everolimus combination therapy were observed to reduce the incidence of [respiratory tract infections (RTIs)] at one year by 42% (p=0.006) and 36% (p=0.01), respectively. [MR: It looks to me like combination therapy is a wash vs. everolimus alone]. The combination of RTB101+everolimus was also observed to significantly enhance the response to influenza vaccination and upregulated the expression of critical antiviral genes that play a key role in enabling the immune system to protect the elderly from respiratory tract infections.
RTIs are the fourth leading cause of hospitalizations and the seventh leading cause of death in people aged 65 years and older in the United States. Moreover, the majority of RTIs in the elderly are caused by viruses for which there are currently no approved therapies. resTORbio’s TORC1 inhibitor program has the potential, if successfully developed and approved, to be a new class of immunotherapy that enhances the function of the aging immune system to fight infectious pathogens including viruses, and thereby reduce the incidence of respiratory tract infections.
Based on the results of the Phase 2a study, resTORbio is conducting a Phase 2b clinical trial to further investigate the potential benefits of RTB101 alone and in combination with everolimus in aging-related diseases. In the ongoing Phase 2b study, doses of RTB101 alone and in combination with everolimus are being evaluated as an immunotherapy to decrease the incidence of RTIs in older people at increased risk of morbidity and mortality from RTIs (defined as age 85 and older and age 65 and older with comorbidities). Dosing has been completed in the Phase 2b study and 16-week topline data are expected to be reported in the third quarter of 2018.
Possible Anti-Aging Drugs Boost Elderly Immune Systems
... After a year, the study subjects who got the two-drug combination reported 1.49 infections, such as colds and bronchitis, the researchers report. Those who took the placebo reported 2.41 infections. That works out to be about a 40 percent reduction.
"That's a big reduction," Mannick says. [MR: on a relative basis, yes — but it's a small absolute effect in a small number of subjects in each arm].
The drugs also boosted how the elderly study subjects' immune systems responded to a flu vaccine — increasing the levels of flu antibodies their immune systems produced by more than 20 percent, Mannick says. ...
The researchers stress that more research is needed to confirm the findings and show the drugs are safe. And at least one researcher says the findings are based on a relatively small number of people and used methods that could produce misleading results. [The article never clarifies what these methods are -MR]
Still, many researchers say the findings are encouraging.
"I think the results are quite exciting," says Felipe Sierra, director of the division of aging biology at the National Institute on Aging. ... "It is possible that further research with this same combination of drugs or similar combinations will lead to a possibility of addressing multiple chronic diseases because these drugs have been shown to affect the process of aging itself," Sierra says. ...
"It is premature, in my opinion, to rule out major negative effects of the pharmacological agents used in this study among sub-sets of human subjects," wrote George Martin, a professor emeritus of pathology at the University of Washington. ...
Aubrey de Grey, an aging researcher at the SENS Research Foundation in Mountain View, Calif., says that "perhaps the most exciting aspect of the results is that the protection lasted for the duration of the study, namely a year, even though the drug was only given for the first six weeks."
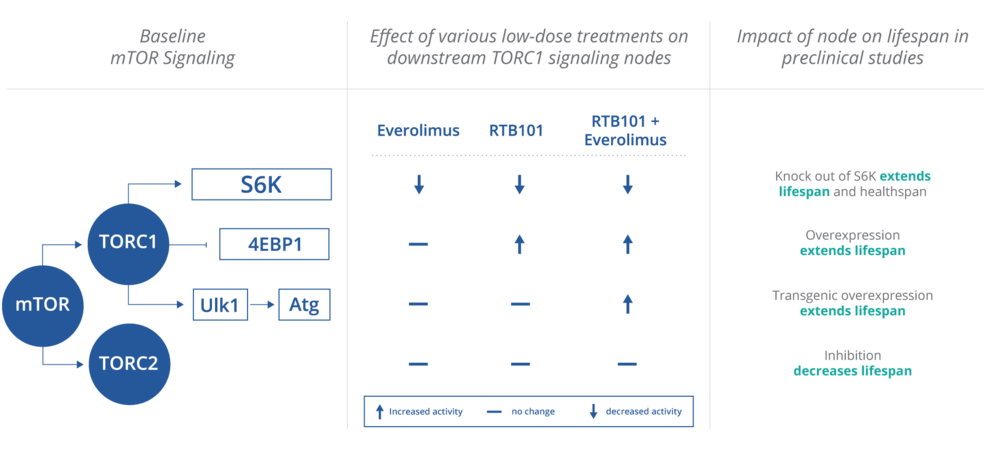
Effects of Everolimus and RTB101/BEZ235 on mTOR Signaling. From resTORbio Website
About RTB101 [resTORbio Website]
RTB101 is an orally administered, small molecule, potent TORC1 inhibitor. RTB101 partially inhibits TORC1 by binding to the active site of mTOR on the TORC1 complex, a mechanism known as catalytic inhibition. [That sounds like classic competitive inhibition to me, but I'm coming at this from no deep reading —MR]. ... Everolimus partially inhibits TORC1 by changing the shape of TORC1, a mechanism known as allosteric inhibition. The combination of RTB101 and everolimus may yield complete and selective TORC1 inhibition.
NVP-BEZ235 is an imidazo[4,5-c]quinoline derivative that inhibits PI3K and mTOR kinase activity by binding to the ATP-binding cleft of these enzymes. In cellular settings using human tumor cell lines, this molecule is able to effectively and specifically block the dysfunctional activation of the PI3K pathway, inducing G1 arrest. The cellular activity of NVP-BEZ235 translates well in in vivo models of human cancer. ...
NVP-BEZ235 significantly reduced the phosphorylation levels of the mTOR activated kinase p70S6K (Fig. 3A). This kinase is activated by the TORC1 complex, which itself is relieved from a negative regulation by the TSC1/2 complex in a PI3K- and Akt-dependent manner (35). Cell line lacking functional TSC complexes (TSC1-null MEF) are characterized by a constitutively active mTORC1 complex (36). As expected, TSC1-null MEF exposed RAD001 showed a complete reduction of S235/S236P-RPS6 levels, even at the lowest concentration tested (1 nmol/L). Under similar experimental conditions, NVP-BEZ235 also led to a reduction of S235/S236P-RPS6 levels with an IC50 of 6.5 nmol/L (Fig. 3C), suggesting that NVP-BEZ235 can directly inhibit the mTOR kinase, as the kinase domain of mTOR is highly homologous to the one of class IA PI3K. The activity of NVP-BEZ235 against mTOR was confirmed using a biochemical mTOR K-LISA assay (IC50, 20.7 nmol/L; Supplementary Fig. S4A).6 To test whether NVP-BEZ235 inhibits both mTORC1 and mTORC2 complexes, immunokinase assays were done using 4E-BP1 (Phas) as a substrate. Maximum activity of TAP-tagged immunoprecipitated Raptor (mTORC1) or Rictor (mTORC2) kinase complexes was reached after an incubation period of 30 min (Supplementary Fig. S4B).6 The addition of NVP-BEZ235 to otherwise similar assay conditions caused a concentration-dependent decrease in mTORC1 and mTORC2 activities (Fig. 3D). Altogether, these data show that NVP-BEZ235 is also a mTORC1/2 catalytic inhibitor.
PMID 18606717





















































