I’ve been trialling a new protocol and have a few things to report. Generally, I’ve been feeling great, good energy, good sleep, but on the downside, I have been experiencing occasional fast heart beats. These are not flutters. They are proper, strong heartbeats that send the blood round my body. Imagine getting a small shock of adrenaline. That is what they feel like. I get these from time to time and to begin with it was unclear whether or not the protocol was making this worse. But it now appears that it is making it worse.
I built the protocol up one supplement at a time in the AM to determine effects, positive or negative.
- First, I introduced phloretin at 30-50mg/day to determine if this made me fatigued (see previous report). At this level it does not make me fatigued. I did notice my fat percentage creeping up again on the machine.
- After 2 days of phloretin this I added Boswellia extract back in.
- After 2 days of Boswellia (on the 2nd day I doubled the dose) I introduced forskolin.
- Forskolin affected my bowel movements and made me very alert for a few days but then things went pretty much back to normal. I noticed it also seems to counter the fat increasing effects of the phloretin.
- I then added artichoke extract to increase the effectiveness of the forskolin.
- After another couple of days I then added Tongkat Ali back in as a second ROCK inhibitor (in addition to phloretin).
- I then changed to taking all the supplements in the evening instead of the morning. Both seem to work equally well and no obvious side effects immediately occurred.
My diastolic blood pressure dropped about 10 points. My systolic blood pressure is inconsistent. My heart rate variability results are good (much better than population average for my age), although I have not been doing this monitoring for long, so I don’t have a previous baseline. I have now noticed during and after exercise that I am experiencing some sharp, spikey heartbeats that the monitor is removing as artifacts. But I know they are real heartbeats as I can feel them. This has not affected my exercise performance in any way, but it is a little disturbing.
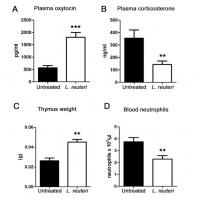
- Last night I injected about 300ug of oxytocin and 10mg of epitalon.
I immediately felt flushed and hot in the face and to a lesser extent on the rest of my skin. This effect diminished after a few minutes. I know this is due to the oxytocin, as I’ve used epitalon lots of times before without this effect. I was quite excited and apprehensive injecting oxytocin so although I was monitoring my heart rate, which was elevated, it was impossible to say whether this was due to the oxytocin or just my excitement.
The next morning I felt normal, maybe a little more fatigued than usual given I hadn’t exercised the night before (although this could be due to the epitalon). Later in the day I took a blood pressure measurement. My diastolic had dropped by another 5 points. The first came back at 143/59. My normal levels are 120/74. The second reading was 107/58.
My best guess is that the combination of the supplements I am using is affecting my heart (and probably other muscles) by inhibiting the actin cytoskeleton and myosin fibres. This might explain the erratic heart beats and wildly varying systolic pressure (the lowered diastolic is expected from my protocol based on previous results). I may be jumping to conclusions but there is some support for the heart to be negatively affected by tgf-b inhibition:
http://basictranslat.../content/4/1/41
The role of the transforming growth factor (TGF)-β pathway in myocardial fibrosis is well recognized. However, the precise role of this signaling axis in cardiomyocyte (CM) biology is not defined. In TGF-β signaling, SMAD4 acts as the central intracellular mediator. To investigate the role of TGF-β signaling in CM biology, the authors deleted SMAD4 in adult mouse CMs. We demonstrate that CM-SMAD4–dependent TGF-β signaling is critical for maintaining cardiac function, sarcomere kinetics, ion-channel gene expression, and cardiomyocyte survival. Thus, our findings raise a significant concern regarding the therapeutic approaches that rely on systemic inhibition of the TGF-β pathway for the management of myocardial fibrosis.
Even if the above paper is the extreme case (i.e. deleting SMAD4), it does indicate a fundamental limit to this method of rejuvenation and indicate I may need to moderate my protocol in either dose or duration. Nothing like this was reported in the Conboy study; their protocol was done for 7 days.
Edited by QuestforLife, 12 February 2020 - 02:22 PM.