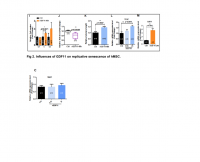
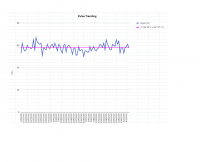
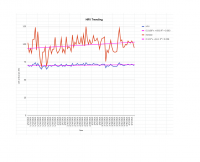
Thanks, I read the study too fast and missed the part where they dosed 12mg (not 24mg) twice. Either way, I've only thus far being taking 1x120mg gotu kola tablet per day, with ~43% triterpenes (~24mg asiaticoside), so according to 'my' study I should be in the right serum range.
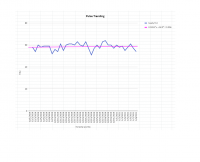
But I am a little alarmed at the much larger plasma concentrations achieved in the study you reference. If those numbers are accurate we'd need a much lower dose (see attached pic, higher curve is after repeated dosing for 6 days).
@:study1 [Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives]@{[mmr-20-04-3701.pdf]}
@:study2 [reference 78] @{[grimaldi1990.pdf]} Grimaldi, R., De Ponti, F., D’Angelo, L., Caravaggi, M., Guidi, G., Lecchini, S., … Crema, A. (1990). Pharmacokinetics of the total triterpenic fraction of Centella asiatica after single and multiple administrations to healthy volunteers. A new assay for asiatic acid. Journal of Ethnopharmacology, 28(2), 235–241. doi:10.1016/0378-8741(90)90033-p
@:study3 @{[rush1993.pdf]} The comparative steady-state bioavailability of the active ingredients of Madecassol
Yeah, I considered that.
As I mentioned
"I can't explain the lack of matching peaks, unless the 30mg triterpene dose in the other study had much more that contributed to the resulting asiatic acid content than ~12-15mg of asiaticoside, or, perhaps, there was something delaying the 12mg dose absorption. "
Well, the study (@{study2}) provides more detail:
"The three principal components of the TTF of C. asiatica are asiatic acid, madecassic acid and asiaticoside. Asiatic and madecassic acids together account for approximately 60% and asiaticoside for 40% of the product. "
So, 30mg dose of TTF is 12mg asiaticoside + ~9mg asiatic acid. In @{Study3}, 6mg of asiatic acid spiked blook concentration to .082mcg. If you model that, on the graph showing the 6mg asiatic acid and the 12mg asiaticoside, the removal of asiatic acid is continual, and then you combine the two results (to replicate the 30mg TTF dose), the .5mcg seen in the other study fits. It's a bit complex as to why:
The trailing end of slower decrease in blood concentration of asiatic acid is probably due to, as I mentioned:
1. Asiaticoside to asiatic acid was inhibited [slower] more highly at lower concentrations of asiatocide.
2. Slow asiaticoside to asiatic acid, particularly at the tail end, was likely also due to latent asiaticoside build up that is released over time (accumulation in fat?)
And one more I didnt mention
3. removal of asiatic acid is slower and lower concentrations
This would mean the max removal of asiatic acid from the blood would need to be calculated prior to build up of asiaticoside, which would be slowly transformed into asiatic acid, decreasing the decrease rate of asiatic acid. The best we can do with the graphs is find the max decrease after the peak, which is about .22mcg/hr (@{study2}). While, on the lower end, from @{study3}, you'd get a removal max of ~.03mcg/hr. Expectably, removal ramps up to a maximum as asaitic acid content increases.
Since both lines are experiencing this asiatic acid reduction (two separate groups), after combining the lines, you have to add the removal rate to get what the combination would look like. The pre-offset peak would be ~.140mcg/ml, at hour 3, compared to @{study1} of a .52mcg/ml peak at hour 4. Considering an asiatic acid removal rate of between .22mcg/hr and .03mcg/hr, favoring the higher rate (since there would be higher asiatic acid content), the studies fit.
So, I will cycle back to things I've said:
1. "Looking up triterpene content in extracts, I estimate 10mg/ml on the high side [Triterpene Composition and Bioactivities of Centella asiatica](
https://www.mdpi.com...9/16/2/1310/htm) - unless explicit isolation.
Assuming ~1g/ml, the standard dosage on supplemental gotu kola is presented as 1g, which fits nicely into the recommendable amount based on my numbers.""
2. Regarding your 2x [now 1x] 24mg asiaticoside dose "Regardless, your dose is too high."
Considering that .2mcg/ml has lower, but still elevated, telomerase activity compared to .02mcg/ml, the target should be .02mcg/ml. In reality, the .02mcg/ml could already be in the decline phase (ie, 0.01mcg/ml could have max telomerase activity), since lower was not measured. Somwhere btween .2mcg/ml and 2mcg/ml is an unacceptable level, where telomerase activity is suppressed.
And, it gets a little more complicated.
@{study1} 08AGTLF is 95% triterpenes (and asiatic acid) (
http://www.apexbt.co...iaticoside.html), not 100% asiaticoside. The other studies @{study2} @{study3} measure asiatic acid, not triterpene content, where as, @{study1} is ~triterpene content.
We might consider that the total blood content of asiatic acid can be used to determine the blood content of asiaticoside. We might consider that the blood content of asiaticoside maxes out shortly after dosing, when the dose is mostly absorbed. As such, we can consider that the asiatic acid after about, lets say, hour 2, represents a constant decrease of blood asiaticoside being transformed into asiatic acid. As such, the area under the curve of asiatic acid (using an asiatic acid removal rate algorithm (to value the x axis time periods based on variable removal rate)), would give you the max blood concentration of asiaticoside (at hour ~0-2).
If we assume 2hr peak and average .1mcg/ml asiatic acid when above .1mcg/ml blood concentration,
from @{study2}
3hr rise to peak after 2hr mark (reducing at .1mcg/ml)
rise until 2hr mark assumed .1mcg/ml per hour reduction
9hr reduction (reducing at .1mcg/ml)
You get, [excuse my sloppiness], ~1mcg/ml max asiaticoside concentration (ignoring the partial asiatic content of TTF) to explain the peak .5mcg/ml asiatic acid blood concentration after a 30mg TTF dose.
IE, I would guess that asiaticoside content will be higher initially than the peak that asiatic acid reaches.
But, considering I take ~1.5mg of asiaticoside (from the 10mg triterpenes in a regular extract), and that the study indicating .02mcg/ml was on a triterpenes mix (not isolated asiaticoside) (ie, 0.02mcg/ml of triterpenes, not asiaticoside), this isn't concerning enough for me to investigate further. All that said, I seem to be allergic to gotu kola... (jk)
Edited by capob, 16 February 2021 - 07:38 PM.