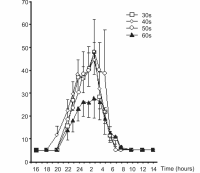
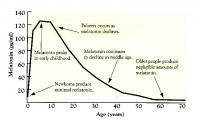
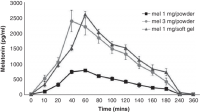
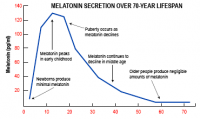
Same, pg/ml
The levels you are getting via supplementation seem insanely high. Unless you're having sleep issues I wouldn't want to mess with those kind of levels.
1mg powder seems to generate about 500 level. That suggests that 500microgram or 300microgram might generate notably less, perhaps half or less of that. Some sources say young humans have around 180 level, so it'd probably be a tiny bit higher than that. But I could be wrong, would need to investigate further to see.
Note sources vary some claim 180 is human peak, others claim it is around 130. But in any case it has a very short half life and is rapidly cleared from the body.
Some highlights from some research on melatonin
A very large body of evidence indicates that melatonin is a major scavenger of both oxygen- and nitrogen-based reactive molecules (49–53), including ONOO−(54–56). Melatonin has scavenging actions at both physiologic and pharmacologic concentrations. Not only melatonin but also several of its metabolites can detoxify free radicals and their derivatives (57–59). Studies also reveal that melatonin eliminates the decomposition products of ONOO−, including OH•, NO2•, and the carbonate radical (CO3•−) in the presence of physiological carbon dioxide concentrations (60–62). Melatonin also supports several intracellular enzymatic antioxidant enzymes, including SOD and glutathione peroxidase (GSH-Px) (63,64). Moreover, melatonin induces the activity of γ-glutamylcysteine synthetase, thereby stimulating the production of another intracellular antioxidant, glutathione (GSH) (65). A number of studies have shown that melatonin is significantly better than the classic antioxidants in resisting free-radical–based molecular destruction. In these in vivo studies, melatonin was more effective than vitamin E (66–68), β-carotene (69), and vitamin C (69–71), and superior to garlic oil (72). Beneficial antioxidant effects of melatonin have been recently shown in clinical settings for several chronic diseases, including patients with rheumatoid arthritis (73), elderly patients with primary essential hypertension (74), and females with infertility (75).
Several antioxidants reportedly preserve the activities of SOD and/or GSH-Px. These effects are indirect, however, owing to their ability to scavenge free radicals and protect the protein from damage. Melatonin, on the other hand, possesses genomic actions and regulates the expression of several genes, including those for SOD and GSH-Px. Melatonin influences both antioxidant enzyme activity and cellular mRNA levels for these enzymes under physiological conditions and during elevated oxidative stress (63), possibly through epigenetic mechanisms (76–78). The occurrence of these two features in a single molecule is unique for an antioxidant, and both actions protect against pathologically-generated free radicals.
We recently reviewed the mechanisms by which melatonin improves mitochondrial respiration and increases ATP synthesis under physiological and poisonous conditions (80). Consequently, the antioxidant and free-radical–scavenging capacities of melatonin protect proteins of the [mitochondria] ETC[electron transport chain] and mtDNA from ROS/RNS-induced oxidative damage. This protective effect limits the loss of intramitochondrial GSH, improves ETC activity, and reduces mtDNA damage. Melatonin’s actions at the mtDNA level also increase the expression of complex IV and the activity of complex I and complex IV of the ETC (81).
Another advantage of melatonin over classical antioxidants is its lack of prooxidative actions. All classical antioxidants are potential electron donors and they exhibit both reduced and oxidized forms. Once they donate an electron to neutralize a free radical, they are transformed from a reduced to an oxidized state. Usually, the oxidized form will be regenerated to the reduced state through the mechanism known as redox reaction or recycling. In this pathway, the recycling of vitamin C or vitamin E occurs at the expense of GSH. In many cases, however, GSH is a better antioxidant than either vitamin C or vitamin E (103). Because these antioxidants are electron donors and exhibit redox reactions, their oxidized forms also can oxidize other molecules. Therefore, classical antioxidants are prooxidants. Melatonin sacrifices itself and does not participate in redox cycling after scavenging free radicals. As previously mentioned, melatonin not only does not consume cellular GSH, it also preserves or even increases the content of GSH in tissues. Thus, melatonin is classified as a suicidal or terminal antioxidant (104).
Melatonin or N-acetyl-5-methoxytryptamine is a highly conserved indoleamine molecule found in all microorganisms (Hardeland and Fuhrberg, 1996), plants and animals (Reiter et al., 2001; Tan et al., 2012). Originally identified in the bovine pineal gland (Lerner et al., 1958), melatonin is also produced by a wide range of tissues including the retina, thymus, spleen, heart, muscle, liver, stomach, pancreas, intestine, placenta, testis, ovaries, bone marrow, skin and hair follicle, cerebral cortex, and striatum (Stefulj et al., 2001; Venegas et al., 2012; Acuna-Castroviejo et al., 2014). The content of melatonin in these tissues varies and decreases with age to a similar extent as its pineal production (Sanchez-Hidalgo et al., 2009; Scholtens et al., 2016).
Although the levels of melatonin in foods are much lower than those of melatonin given as a nutritional supplement, consumption of foods rich in melatonin significantly increases circulating melatonin levels in the range of the physiological concentrations (Maldonado et al., 2009; Johns et al., 2013; Sae-Teaw et al., 2013). However, little is known about the influence of the dietary melatonin intake on human health. In this review, evidence for cardiovascular health benefits of endogenous melatonin and melatonin supplementation as a pharmacological agent or from the diet is discussed.
A strong inverse relationship exists between endogenous melatonin levels and cardiovascular disease (Dominguez-Rodriguez et al., 2010). Epidemiological studies report that both nocturnal melatonin synthesis and circulating levels are reduced in patients with coronary heart disease (Brugger et al., 1995; Altun et al., 2002; Dominguez-Rodriguez et al., 2002), hypertension (Kozirog et al., 2011; Dominguez-Rodriguez et al., 2014), heart failure (Girotti et al., 2003; Dzida et al., 2013; Kimak et al., 2014; Dominguez-Rodriguez et al., 2016) and cardiovascular risk conditions such as diabetes (McMullan et al., 2013) and obesity (Mantele et al., 2012). Incidence for adverse cardiac events, including myocardial infarction (Dominguez-Rodriguez et al., 2002), sudden cardiac death (Muller et al., 1987) and cardiac arrhythmias (Siegel et al., 1992) increases in the early morning, when circulating melatonin levels are considerably lower (Altun et al., 2002). Similarly, low melatonin secretion levels are associated with a greater risk of incidence for myocardial infarction in women with increased body mass index (McMullan et al., 2017), supporting the crucial role of endogenous melatonin in cardiovascular pathologies.
Melatonin has also been shown to improve survival rates in patients with prostate and colorectal cancer. Studies have shown that men with primary localized malignant prostate tumors have extremely low levels of nocturnal melatonin that decrease with an increase in tumor growth. [46] Patients with unoperated colorectal carcinoma were found to have a significantly lower nocturnal plasma melatonin level compared to controls. [47] However, a second study conducted by Kvetnaia et al. found a higher nocturnal urinary metabolite 6-sulfatoxymelatonin (aMT6s) excretion in operated, untreated males. In one study of 54 patients with metastatic lung and colorectal tumors, the melatonin regimen resulted in stabilization of cancer and improved quality of life for roughly 40% of the recipients. [31, 48] As specified previously, the effects of melatonin supplementations can change according to the time of administration. Melatonin injections given in the morning have been found to stimulate cancer growth while injections in the evening contribute to tumor regression. Afternoon injections have no apparent effect. [31]...
Although melatonin has been successfully used in cancer and sleep disorder treatments, improper timing of use can result in negative outcomes. Melatonin injections in the morning can stimulate tumor growth; doses in the afternoon exhibit no effect, and doses in the evening have retarding effects. [31] Animal studies have shown that large doses of melatonin increased light-induced damage to retinal photoreceptors (ganglion cells, rods, and cones).
Melatonin doesn't last in the body for long. It has a half-life of 40 to 60 minutes. The half-life is the time it takes for the body to eliminate half a drug.Sep 11, 2019-healthline.com
attached another graph of melatonin release from another source, the following link:
Two animal studies showing benefits at both high and low dose, but some side effects at high dose.
From the age of 3 months until their natural death, female Swiss-derived SHR mice were given melatonin with their drinking water (2 or 20mg/l) for 5 consecutive days every month. Intact mice served as controls. There were 54 mice in each group. The results of this study show that the treatment of melatonin did not significantly influence food consumption, but its administration at lower doses did decrease the body weight of mice; it slowed down the age-related switching-off of estrous function; it did not influence the frequency of chromosome aberrations in bone marrow cells; it did not influence mean life span; and it increased life span of the last 10% of the survivors in comparison to controls. We also found that treatment with low dose melatonin (2mg/l) significantly decreased spontaneous tumor incidence (by 1,9-fold), mainly mammary carcinomas, in mice whereas higher doses (20mg/l) failed to influence tumor incidence as compared to controls. For this reason, we conclude that the effect of melatonin as a geroprotector is dose-dependent.
https://pubmed.ncbi....h.gov/12670632/
From the age of 6 months until their natural deaths, female CBA mice were given melatonin with their drinking water (20 mg/l) for 5 consecutive days every month. Intact mice served as controls. The results of this study show that the consumption of melatonin did not significantly influence food consumption, but it did increase the body weight of older mice; it did not influence physical strength or the presence of fatigue; it decreased locomotor activity and body temperature; it inhibited free radical processes in serum, brain, and liver; it slowed down the age-related switching-off of estrous function; and it increased life span. However, we also found that treatment with the used dose of melatonin increased spontaneous tumor incidence in mice. For this reason, we concluded that it would be premature to recommend melatonin as a geroprotector for long-term use.
Yeah the attached graph doesn't look too pretty, and I don't think I'd want something with so many beneficial effects declining significantly in my body. Assuming any truth to the graph
Edited by Castiel, 19 May 2021 - 05:59 PM.