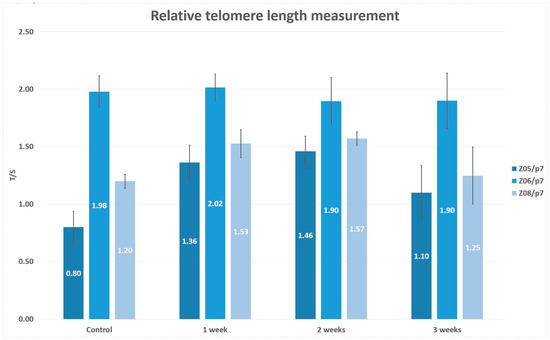
We should be aiming for mouse cell levels of telomerase, not HELA levels
Most mammalian cells do not divide indefinitely, owing to a process termed replicative senescence. In human cells, replicative senescence is caused by telomere shortening, but murine cells senesce despite having long stable telomeres1. Here, we show that the phenotypes of senescent human fibroblasts and mouse embryonic fibroblasts (MEFs) differ under standard culture conditions, which include 20% oxygen. MEFs did not senesce in physiological (3%) oxygen levels, but underwent a spontaneous event that allowed indefinite proliferation in 20% oxygen. The proliferation and cytogenetic profiles of DNA repair-deficient MEFs suggested that DNA damage limits MEF proliferation in 20% oxygen. Indeed, MEFs accumulated more DNA damage in 20% oxygen than 3% oxygen, and more damage than human fibroblasts in 20% oxygen. Our results identify oxygen sensitivity as a critical difference between mouse and human cells, explaining their proliferative differences in culture, and possibly their different rates of cancer and ageing. source: https://www.ncbi.nlm...les/PMC4940195/
This paper really got me thinking.
Mouse cells senesce quickly at 20% oxygen but not at all at 3% (roughly physiological levels). Human cells senesce more slowly than mouse cells at 20% due to better defence against ROS, but unlike mouse cells do (eventually) senesce at 3% oxygen levels, due to lack of telomerase. The difference between 20% and 3% oxygen acts as growth inhibition (see my recent post on epitalon and T cells). The normal state of most human cells is (near) hypoxia (1-6%).
Most mammalian tissue cells experience oxygen partial pressures in vivo equivalent to 1–6% O2 (i.e., physioxia). In standard cell culture, however, headspace O2 levels are usually not actively regulated and under these conditions are ~18%. This drives hyperoxia in cell culture media that can affect a wide variety of cellular activities and may compromise the ability of in vitro models to reproduce in vivo biology. source: https://www.hindawi....l/2018/8238459/
Bill Andrews uses HELA cancer cell telomerase levels as his benchmark for stopping aging in human cells. But given the above, I think a better benchmark would be differentiated mouse cells. But finding out what these telomerase levels are has not been easy. Eventually I found a thread of evidence linking HELA and Mouse telomerase levels.
FIG 1.Telomerase activity in human (cancer) cell lines:

Source: https://www.nature.c...rticles/1206468
Note that HELA levels are slightly above U937 (leukaemia cells line) telomerase levels.
Fig 2. Bottom Left: TRAP Assay(telomerase protein level) of HELA and K562 cells

Source: https://www.cell.com...t/S0092-8674(00)80538-3
Note that HELA levels are slightly less than K562 (another cancerous leukaemia line) telomerase levels.
Next I found a link showing the telomerase level of K562 and HL60 (yet another leukaemia cells line) is the same:
Fig 3. mRNA TERT (top left) and protein telomerase (top right) white panels for HL60 and K562 cell lines

source: https://bmccancer.bi...471-2407-11-512
Finally I tied it all together by finding a paper comparing HL60, U937 and both differentiated and undifferentiated mouse cells:
Fig 4. TOP LEFT: mRNA TERT levels for HL60 and U937 (cancer), H9 (human stem) and Mouse (stem white and grey differentiated) cells

source: https://academic.oup.../8/2618/2410230
This last paper is interesting in its own right, showing how human stem (H9) or cancer cell lines (HL60 and U937) lose most (by a factor of 37-1000) or all of their telomerase expression when they differentiate, whereas mouse cells by comparison drop only by a factor of 5-10.
But for our purposes we can now link HELA telomerase levels to the telomerase levels of U937 or HL60 (via a comparison with K562) cells to that of differentiated mouse cells. From Fig 1 we can see HELA telomerase levels are a little above that of U937 and from Fig 2 a little below that of K562 and (through Fig 3) HL60 cells. For the sake of argument this puts HELA at about the same level as Human embryonic stem cells (H9) or undifferentiated mouse cells (M1 or F2-1 types) in Fig 4. This is 5 or 6 times higher than differentiated mouse cells (grey bars in same image).
Thanks for sticking with me. I did find other references confirming the comparations in telomerase levels I have shown above. I am reasonably happy I'm not cherry picking my data.
If the above is even vaguely accurate, then for human cells in 3% oxygen, we can reduce our telomerase requirement by 5 or 6 times. If TAM-818, Bill Andrew's telomerase activator, is 16% of HELA (as he states), then it is 83-94% of differentiated mouse cells' telomerase levels (depending on whether you are talking about M1 or F2-1 cells; it's even better for F2-9 cells). This is very close to what we need to stop aging.
You might argue the assumption all our tissues are at 3% oxygen is not valid; I quoted a reference stating 1-6% above. I plan to make up that shortfall by periodically increasing MTOR inhibition, which is (in my opinion) the main effect of hypoxia.
Edited by QuestforLife, 11 June 2021 - 09:24 AM.