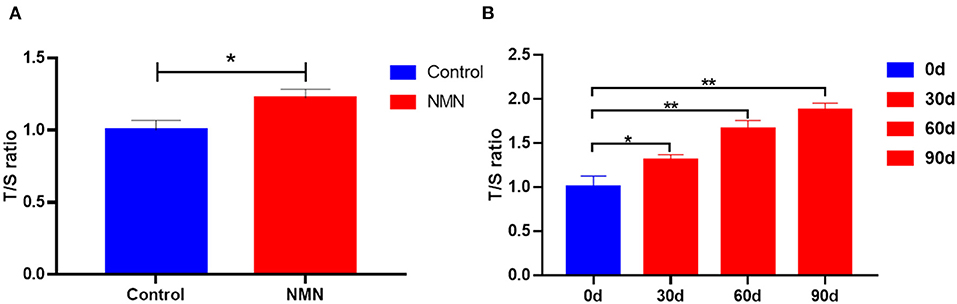
Comment on gonadal rejuvenation by GDF11
Abstract: Growth differentiation factor 11 (GDF11), also known as bone morphogenetic protein 11, has been shown to have rejuvenation and antiaging properties, but little information is available regarding the role of GDF11 in reproductive system to date. In this study, we first confirmed the bioavailability of recombinant GDF11 (rGDF11) by oral delivery in mice. We also showed that dietary intake of rGDF11 had little influence on body and gonadal (ovary/testis) weights of recipient mice, indicating their general condition and physiology were not affected. Based on these findings, we started to test the function of rGDF11 in ovary and testis of mice and to explore the underlying mechanisms. It was found that to some extent, rGDF11 could attenuate the senescence of ovarian and testicular cells, and contribute to the recovery of ovarian and testicular endocrine functions. Moreover, rGDF11 could rescue the diminished ovarian reserve in female mice and enhance the activities of marker enzymes of testicular function (sorbitol dehydrogenase and glucose-6-phosphate dehydrogenase) in male mice, suggesting a potential improvement of fertility. Notably, rGDF11 markedly promoted the activities of antioxidant enzymes in the ovary and testis, and remarkably reduced the levels of lipid peroxidation, protein oxidation, and reactive oxygen species (ROS) in the ovary and testis. Collectively, these results suggest that GDF11 can protect ovarian and testicular functions of aged mice via slowing down the generation of ROS through enhancing activities of antioxidant enzymes.
https://doi.org/10.1093/gerona/glab343
With thanks to dlewis1453 for acquiring and sharing the paper with me. I have added some personal notes on my experiences with GDF11 within the text.
The paper is somewhat obscure. The research was carried out in China and published by Oxford University Press on behalf of the Gerontological Society of America. The main aim of the work was to look at the effect of exogenous GDF11 on markers of reproductive health in aged male and female mice. The authors were also previously responsible for developing a highly specific GDF11 antibody, which has proven the decline of GDF11 with age (there was some controversy over this because of its similarity with GDF8).
Of note, the increase of circulating GDF11 achieved in these mice was from 500 pg/ml to 630 pg/ml, a increase not likely to stimulate GDF8 receptors (personal note: I can no longer inject GDF11 without sharp increases in BP; presumably my levels are now considerably elevated from normal).
Remarkably the authors injected the recombinant GDF11 into normal mouse feed and dosed them orally at ~1mg/kg/day for 4 weeks and it was bioavailable, as shown by increases in serum levels at 3 and 6 hours after feeding, but removed by 18 hours. The mice fed GDF11 were 12 months old (middle aged). I find it remarkable what improvements were found in only a 1 month study. I wonder if a supplement manufacturer will take the leap and produce an oral GDF11 supplement?
No change in body weight was observed using GDF11 (note I experience an increase in appetite and an increase in weight, injecting GDF11).
50% of control female mice had irregular cycles (the precursor to menopause in human females), but only 33% of the GDF11 dosed group did. Estradiol and progesterone had declined markedly in aged female mice but this was mostly restored in the mice treated with GDF11. Note: this was not treating mice all their lives to prevent these changes; it was treating already middle aged mice to restore functionality. Relevant to this, active/growing follicle count was increased by GDF11 administration. Looking at the health of egg cells in detail, control mice oocytes had swollen mitochondria and lipid accumulation in the cytoplasm. GDF11 mice had smaller mitochondria with visible, functioning cristea, and reduced lipid drop accumulation.
Moving onto male mice, aged male mice had only one third of the testosterone level of young mice; mice treated with GDF11 were around halfway in between the two (personal note: I am more aggressive the day after injecting GDF11). Looking at various RNAs, it appeared that GDF11 was working via stimulating testosterone production. Sorbitol dehydrogenase (SDH) and glucose-6-phosphate dehydrogenase (G6PD) are important enzymes for testosterone production and they were upregulated by GDF11.
Lipofuscin accumulation in the testes and ovaries of aged male and female mice increased in controls compared to young mice, but was reduced somewhat by GDF11.
Lipid peroxidation and protein oxidation were decreased in the ovaries and testes of treated male and female mice compared to controls.
Glutathione peroxidase, Superoxide dismutase and catalase were elevated in the ovaries and testes of GDF11 treated mice compared to controls, and overall ROS levels were reduced.
It is interesting that the authors found that GDF11 was so effective at decreasing ROS. Though most ROS centric theories look at direct damage from ROS, I expect future studies will find an epigenetic connection via demethylases (I have found AKG to hugely decrease the amount of GDF11 I can tolerate).
I have attached several screenshots of figures from the paper if the reader wants to verify some of the statements I have made above.
Edited by QuestforLife, 04 February 2022 - 10:37 AM.