Hi Quest, how is your recent protocol going? Your recent posts made strong cases for adding nucleotides, manganese, selenium, and CoQ10 to your protocol. I recall you were also taking ROCK inhibitor eye drops sublingually.

Alternative methods to extend telomeres
#991
Posted 30 April 2024 - 08:35 PM
#992
Posted 01 May 2024 - 02:37 PM
In short and in reverse order:
Rock-inhibitor eye drops: these probably contributed to my sudden epigenetic age recovery, as reported here. I stopped taking them however, as they caused the back of my throat to become slightly swollen and I started snoring/snorting at night.
CoQ10/Selenium: I have tried selenium drops, they made me sleepy, so only worth taking at night. It would be worth taking them with a telomere test, when one becomes available.
Manganese: I got too worried about manganese toxicity to continue this; my next blood test after taking manganese for a while did have higher than usual ferritin.
Nucleotides: I saved the worst for last. I added nucleotides to a round* of epitalon and TAM-818. Manganese also made a short appearance at the start of this protocol but was dropped early (see above) Side note: previous to this I had been taking some pollen granules as a way of innoculating myself against hayfever in the coming season. As soon as I started this telomerase activation round I got a case of hives. I am guessing the extra telomerase caused lots of mast cells to be formed. Indeed, a blood test showed elevated eosinophils. It took a couple of months for this to calm down. Perhaps this should be taken as evidence that nucleotides (and potentially manganese) increase telomerase.
(* every other day for 10 days)
Edited by QuestforLife, 01 May 2024 - 02:38 PM.
#993
Posted 01 May 2024 - 03:28 PM
Thanks for the update! Do you have any plans to cycle the rock inhibitor eye-drops?
sponsored ad
#994
Posted 02 May 2024 - 09:33 AM
Thanks for the update! Do you have any plans to cycle the rock inhibitor eye-drops?
I don't remember the names right now (I can find them out later if you want), but I got two different Rho-kinase inhibitor eye drops, which had different concentrations and I think might have been a different ROCK inhibitor too. The stronger (concentration) one had such a strong taste I avoided it, and although it may have been placebo/nocebo, I also felt worse on it. But the weaker one I could put a drop or two under my tongue every night no problem. It was only when I started getting a swollen uvula (dangly bit at back of throat) that I had to stop it. At some point I may use the weaker rock inhibitor again.
At this point I need to have a think about the pros and cons of activating the immune system. My recent brush with an immune reaction has taught me some caution. I have mentioned this in posts before, but the human body seems to maintain a sub-ideal number of WBCs in terms of warding off illnesses; perhaps there are trade offs in play in order to avoid auto-immunity. Activating telomerase shouldn't necessarily result in more cell getting made, but it often does, probably because the body is constantly fighting off infection. It is hard to know where the balance is between, say, making sure you have a healthy replacement pool of endothelial cells for your cardiovascular system, and your immune system going into overproduction mode because you've stupidly ingested some pollen seeds (me).
I do feel like we have a handle now on how to maintain telomeres in the fast turn over cells, if not yet the underlying stem cell cache.
#995
Posted 02 May 2024 - 02:58 PM
I don't remember the names right now (I can find them out later if you want), but I got two different Rho-kinase inhibitor eye drops, which had different concentrations and I think might have been a different ROCK inhibitor too.
Yes, if you could share the names, that would be appreciated. I may want to include them in my new protocol.
At this point I need to have a think about the pros and cons of activating the immune system.
Have you looked into Quercetin for calming allergic responses? It is a natural anti-histamine that seems effective and is also a mild inhibitor of dnmt and hdac. For gently stimulating the immune system I take the heat killed cells of lactoccocus lactis strain plasma, since research shows it is able to directly activate plasmacytoid dendritic cells and thereby stimulate both innate and adaptive immune function.
I do feel like we have a handle now on how to maintain telomeres in the fast turn over cells, if not yet the underlying stem cell cache.
Would this be through a combination of increasing NAD+ and taking selenium, rather than through telomerase activators?
#996
Posted 03 May 2024 - 10:26 AM
Yes, if you could share the names, that would be appreciated. I may want to include them in my new protocol.
Rho Kinase inhibitors
As stated these are eye drops, taken sublingually.
- Netarsudil Opthalmic Solution 0.02% ('Netasudil')
- Ripasudil Eye Drops 0.4% ('Ripatec')
These are potent medicines. Use them at your own risk.
Have you looked into Quercetin for calming allergic responses? It is a natural anti-histamine that seems effective and is also a mild inhibitor of dnmt and hdac. For gently stimulating the immune system I take the heat killed cells of lactoccocus lactis strain plasma, since research shows it is able to directly activate plasmacytoid dendritic cells and thereby stimulate both innate and adaptive immune function.
Quercetin: pros and cons
Yes, I used it to help calm down the itching, and it worked.
But I'd caution against using this for too long; it is a senolytic after all. I found my tendons feeling weak after a week of using it every morning and I got a little bit of tinnitus for a few days, which was terrifying, although could have been unrelated. Quercetin has a short half life, so some people take it multiple times a day, but I wouldn't recommend this.
Would this be through a combination of increasing NAD+ and taking selenium, rather than through telomerase activators?
Main telomerase activation protocol
No, my most recent telomerase-activation protocol was epitalon+TAM818+ the other supplementaries l've already mentioned to enhance the effect (every other day for 10 days).
My experience is this certainly works to keep you free of illness. I advocate doing this now and again, not all the time. As you get the feeling of being depleted otherwise, like when I got low in glutamine. Also, as discussed, you can cause immune reactions in some circumstances. So, I intend to repeat this every winter.
My NMN/Leucine/B6 Protocol
The other approach is the one that doesn't actually activate telomerase (as far as I am aware), but markedly reduces telomere attrition/cell division. This is to use NAD+ precursors. I did some research and found a good combination to be NMN+leucine+B6. The research was done to help people lose weight, but it is good for longevity too. Note: I didn't lose weight on it but did feel benefits (see below).
The basic premise is that the leucine makes it easier for the sirtuins to be activated by NAD+ by lowering the energy hump required for NAD+Sirtuin interaction . This means you can take much lower doses of the NAD+ precursors (I took 250mg of NMN, but you could probably go lower or try other NAD+ precursors. They suggest this makes resveratrol activate sirt1, even though its biovailability is low).
B6 acts with the leucine to reduce fat storage and increase energy utilisation from fat.
The does of leucine is too low to activate mTOR.
I should write a longer post devoted to this, but I don't have time right now.
But I will say this combination made me look younger straight away, and my energy was good. The only issue was that eventually my tendons felt weak, and I hurt my knee doing martial arts, as often happens to me on NAD+ precursors if I don't take decent breaks (or senolytics, see above).
So, right now I am on an extended break from the NMN/B6/Leucine protocol.
References for the NMN/Leucine/B6 protocol for those interested (the second paper has dosage suggestions)
1. Zemel MB. Modulation of Energy Sensing by Leucine Synergy with Natural Sirtuin Activators: Effects on Health Span. J Med Food. 2020 Nov;23(11):1129-1135. doi: 10.1089/jmf.2020.0105. Epub 2020 Aug 4. PMID: 32758058.
2. Zemel MB, Bruckbauer A. Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects. Nutrients. 2012 Jun;4(6):529-41. doi: 10.3390/nu4060529. Epub 2012 Jun 15. PMID: 22822451; PMCID: PMC3397351.
3. Niu KM, Bao T, Gao L, Ru M, Li Y, Jiang L, Ye C, Wang S, Wu X. The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase. Front Nutr. 2021 Nov 29;8:756243. doi: 10.3389/fnut.2021.756243. PMID: 34912838; PMCID: PMC8667784.
Edited by QuestforLife, 03 May 2024 - 10:31 AM.
#997
Posted 13 May 2024 - 05:26 AM
My NMN/Leucine/B6 Protocol
The other approach is the one that doesn't actually activate telomerase (as far as I am aware), but markedly reduces telomere attrition/cell division. This is to use NAD+ precursors. I did some research and found a good combination to be NMN+leucine+B6. The research was done to help people lose weight, but it is good for longevity too. Note: I didn't lose weight on it but did feel benefits (see below).
The basic premise is that the leucine makes it easier for the sirtuins to be activated by NAD+ by lowering the energy hump required for NAD+Sirtuin interaction . This means you can take much lower doses of the NAD+ precursors (I took 250mg of NMN, but you could probably go lower or try other NAD+ precursors. They suggest this makes resveratrol activate sirt1, even though its biovailability is low).
B6 acts with the leucine to reduce fat storage and increase energy utilisation from fat.
The does of leucine is too low to activate mTOR.
I should write a longer post devoted to this, but I don't have time right now.
But I will say this combination made me look younger straight away, and my energy was good. The only issue was that eventually my tendons felt weak, and I hurt my knee doing martial arts, as often happens to me on NAD+ precursors if I don't take decent breaks (or senolytics, see above).
So, right now I am on an extended break from the NMN/B6/Leucine protocol.
References for the NMN/Leucine/B6 protocol for those interested (the second paper has dosage suggestions)
1. Zemel MB. Modulation of Energy Sensing by Leucine Synergy with Natural Sirtuin Activators: Effects on Health Span. J Med Food. 2020 Nov;23(11):1129-1135. doi: 10.1089/jmf.2020.0105. Epub 2020 Aug 4. PMID: 32758058.
2. Zemel MB, Bruckbauer A. Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects. Nutrients. 2012 Jun;4(6):529-41. doi: 10.3390/nu4060529. Epub 2012 Jun 15. PMID: 22822451; PMCID: PMC3397351.
3. Niu KM, Bao T, Gao L, Ru M, Li Y, Jiang L, Ye C, Wang S, Wu X. The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase. Front Nutr. 2021 Nov 29;8:756243. doi: 10.3389/fnut.2021.756243. PMID: 34912838; PMCID: PMC8667784.
Very interesting, great find QuestforLife! I am very curious, how did you look younger from this combination?
The weak tendons and knee injury is unfortunate. You might have induced excessive mitochondrial fission. That is another reason why it may make sense to cycle this protocol. Perhaps you could spend less time off the protocol if you take supplements to induce mitochondrial fusion during the off period.
I found another paper by the same author, "Leucine Modulates Mitochondrial Biogenesis and SIRT1-AMPK Signaling in C2C12 Myotubes" (https://www.ncbi.nlm...les/PMC4220583/) This paper states that HMB has a similar effect as leucine. HMB is a metabolite of leucine that helps to preserve muscle mass during periods of calorie deprivation or physical inactivity. I take it to preserve muscle mass during a cut.
#998
Posted 14 May 2024 - 01:52 PM
Very interesting, great find QuestforLife! I am very curious, how did you look younger from this combination?
Hard to say, it is just the difference between 30 something skin and 40 something skin. Nothing mind blowing but immediately noticeable.
[quote] The weak tendons and knee injury is unfortunate. You might have induced excessive mitochondrial fission. That is another reason why it may make sense to cycle this protocol. Perhaps you could spend less time off the protocol if you take supplements to induce mitochondrial fusion during the off period. [/quote]
Exactly, reduce mitochondrial mass too much and you'll have problems. I think tendons and/or ligaments have less mitochondria, so end up being more vulnerable than say muscles. Conversely, too much time in fusion and you'll be strong and robust, but fatigued and older looking (in my experience). Its a fine balancing act. I don't think you could push towards fission and then back to fusion in one day. I think you'll be in benefits territory either way from days 1-3 or 4 maybe, with the downside coming in after that, but that's only a guess at this point and it may be dependent on the individual state or proclivity towards anabolism vs catabolism.
#999
Posted 11 June 2024 - 11:02 PM
High dose cycloastragenol achieves full age reversal in mice. Senolytic effect was also observed . In this experiment mice were aged prematurely using total body irradiation, until they displayed an aged phenotype. From there they were treated with cycloastragenol at the dose of 50mg per kilogram for two weeks , then sacrificed one week later. The results observed include full reversal of gray hair, return of their mobility/athletic capabilities, return of intestine tissue density and bone density.
https://www.ncbi.nlm...es/PMC10095196/
In this study, we screened a series of compounds reported to have antiaging effects with the aim of identifying new senolytic agents with efficacy and safety both in vitro and in vivo (Figure 6). As a result, we identified CAG, a secondary metabolite isolated from Astragalus membrananceus, as a new, natural, and potent senolytic, which selectively induces cell death in SCs but not NCs. It is noteworthy that CAG also suppresses SASP expression, meaning it can act as a senomorphic to reduce the impact of SCs on the age-related phenotype. Increasing evidence shows that CAG has a wide spectrum of pharmacological functions, including telomere elongation, telomerase activation, anti-inflammatory effects, and antioxidative properties [17].
Edited by marcobjj, 11 June 2024 - 11:04 PM.
#1000
Posted 12 June 2024 - 10:56 AM
High dose cycloastragenol achieves full age reversal in mice. Senolytic effect was also observed . In this experiment mice were aged prematurely using total body irradiation, until they displayed an aged phenotype. From there they were treated with cycloastragenol at the dose of 50mg per kilogram for two weeks , then sacrificed one week later. The results observed include full reversal of gray hair, return of their mobility/athletic capabilities, return of intestine tissue density and bone density.
Thanks for posting the study. Having read it, I am somewhat less bullish about it than you are.
My top-line: I absolutely will not be going out and taking high dose CAG for antiaging purposes.
A couple of cautionary observations before we get into the paper:
All Chinese team – do we trust it? I’ve read Chinese papers before that seemed very promising but no replication ever occurred.
They set out to find senolytics (see Abstract). They already believe (as stated in Abstract) it is senescent cells that are causative in ageing and it is implied that removing senescent cells from mice will translate to humans for anti-ageing purposes. Do we believe these assumptions? On the translation question, my chief concern would be that lab mice can replace lost cells much easier than people, due to their super long telomeres. Somewhat allaying this concern is that cyloastragenol (CAG) is a (weak) telomerase activator.
CycloAstragenol. This is typically taken by humans in the range of 5-20mg/day. But the dose used here would translate to 300mg/day for a human (admittedly they only did it for 2 weeks to mice so we would need, what: 6 months?). Cycloastragenol is also less bioavailable in humans than mice (I read this in Geron documents), which is why I suspect TA-65 is a related but not identical molecule. So ideally if we wanted to dose this properly, we'd want to find a trusted cycloastragenol powder source and encapsulate it in liposomes.
Other technical concerns for the in vitro part (cell study) of the paper:
- Cell choice: IMR-90 and HELF: both human lung fibroblasts; why not a skin fibroblast (NHDF)?
- Stupid concentrations in vivo: 100uM for senolytic effect. Really? Cycloastragenol in human blood reaches ng range, if that. So how do we know that CAG isn’t exercising senolytic action simply by poisoning cells, which the senolytic cells are less resistant to?
- Contrary mechanisms: I’m pretty sure I’ve read that CAG’s activation of telomerase was via AKT (TBC), yet here they say this pathway is inhibited and this is part of its senolytic action. This makes me think these doses would be so high, and so toxic that you wouldn’t get telomerase activation in normal cells, although this remains to be confirmed or refuted. But to me this looks like two completely different mechanisms, with up to 50uM being even slightly beneficial for SCs (perhaps elongating telomeres) but with higher doses killing them. Note: they didn't bother looking at telomerase activation or telomerase elongation from CAG.
- Reporting only what they want to: for the different cell types, they often give the results for different proteins. One is left with the feeling they are presenting what they want to present.
My general feeling from the in vivo portion of the paper is to be unimpressed. But now we move on to the in vitro portion.
The in vivo results are pretty impressive. Impressive in that I didn't realise you could mess mice up that badly and then restore them. But we need to look carefully at what they did. They irradiated the mice until they were pretty sick. Then they gave them massive CAG doses, and remarkably mostly restored them to health. But this isn’t age reversal. This is giving mice a big burden of senescent cells via radiation, and then removing that burden. The mice then replace all those dead cells with new healthy cells, hence the benefits. What would happen if we did this to humans? The nearest equivalent I can think of would be to treat those who have had extensive chemotherapy. Interestingly, chemo survivors are often fine unless they put on weight, when they often develop frailty. I’ve always thought that was interesting and put it down to the proliferation of new fat cells exhausting the almost empty bone marrow reserves. If we treated humans with high CAG doses (assuming we could get bioavailability), could we fix chemo-induced frailty? If we could it would suggest they still have plenty of stem cells, and the frailty is caused by the presence of senescent cells. If we can't it means the stem cells really were depleted. But even if we could, it doesn't really mean we've reversed ageing, just as they didn't reverse ageing in these mice, just restored radiation sickened mice.
Edited by QuestforLife, 12 June 2024 - 10:58 AM.
#1001
Posted 13 June 2024 - 01:23 AM
good points all around but I don't think you can quite compare what was done with the total body irradiation and chemo patients.
I actually have an anectotal about this. My late auntie had intestinal cancer in the early 2000s and she was treated with aggresive radiotherapy using some old school ratiation machines. She survived.
Years later, in 2020 she was hospitalized treat intestinal adhesions. She was put on the operation table and as soon as they opened her up the doctor declared that she was done. Her intestinal tissues have gotten so thin by then, that any attempt to pull and undo the adhesions was destroying the tissue. Which implies the stem cells becoming so spent from the agressive adiotherapy that the lining became to thin to the point it wasn't able to recover from surgery.
The total body irradiation is a generalized and more extreme version of that where just about every single stem cell in the body becomes so exhausted that it displays an early aging phenotype.
And yes you would think that China has a conflict of interest with the cycloastragenol thing. The experiment can be n1 replicated by some of the most intrepid immortalists here, ordering a good batch from Ali and slowly cranking up the dose if no toxic effects are oberved.
But even if we could, it doesn't really mean we've reversed ageing, just as they didn't reverse ageing in these mice, just restored radiation sickened mice.
disagree because I think that any damage will contribute with an uptick in aging, and continued exposure to damage will result in an early aged phenotype. Hence pro-boxers display early onset dementia 10 to 15 years post retirement. Or people with tons of sun exposure displaying and aged skin phenotype. The stem cells naturally run their course and run out of divisions, but punches to the head, UV radiation , radiation therapy simply accelerate that process. They are still all components of aging and not a different problem. Total body irratiation aging is aging nonetheless.
Edited by marcobjj, 13 June 2024 - 01:41 AM.
#1002
Posted 13 June 2024 - 08:31 AM
disagree because I think that any damage will contribute with an uptick in aging, and continued exposure to damage will result in an early aged phenotype. Hence pro-boxers display early onset dementia 10 to 15 years post retirement. Or people with tons of sun exposure displaying and aged skin phenotype. The stem cells naturally run their course and run out of divisions, but punches to the head, UV radiation , radiation therapy simply accelerate that process. They are still all components of aging and not a different problem. Total body irratiation aging is aging nonetheless.
I was probably overly harsh with the paper because of the way they presented things. I do agree it is an interesting result and stem cell exhaustion is clearly an important component of ageing, we can agree.
I am still not certain an aggressive senolytic protocol would be as beneficial for humans as mice; your aunt's story is interesting and very relevant: was the depletion of intestinal lining because stem cells were killed by chemo? Or was it that senescent cell numbers blocked the division of stem cells? Or did senescent cells drive excessive division of (stem) cells and exhaust them this way? Which of these mechanisms is dominant will decide what treatment course is preferable. My intuition is saying that humans have much less senescent cells than mice (as a percentage) and that because our telomeres are much shorter than lab mices', that the exact balance of how we age and what treatment is best to counteract it will be different to mice. Hence why I think an effective telomerase activator for humans is an essential part of any rejuvenation regime.
BTW I should thank you for spurring me to look again at recent telomerase research on pubmed. What a bonaza! So many papers on this subject are coming out at the moment. It seems that telomere and telomerase research as pertains to ageing is undergoing a renaissance. The pick of the bunch from 2024 (so far) is this paper [1]. It is currently behind a paywall and not yet on sci-hub, so I have only read the free sections (I'll review it fully when I have it). But still, it promises to solve something very important...
Firstly, some background. It's long been a mystery to me how sirtuin activators like resveratrol, NMN and potentially Nicotinamde lead to longer telomeres. There have been some papers linking resveratrol to telomerase activation but none are totally convincing (or replicated). And for NMN it really does appear to be a telomerase independent mechanism [2]. I have speculated (for example, here [3]) that the improvement in mitochondrial function might lead to reduced demand on mitochondrial telomerase through reduction in oxidative stress, and therefore lead to a reduction in telomere shortening rate per division, which will show up as long telomeres in downstream replicating cells like leukocytes.
This new paper advances this theory considerably by showing SIRT1 associates with the telomerase protein and both stabilises it and causes nuclear localisation, I.e. moves it into the nucleus, which is likely to make it more effective at lengthening telomeres (SIRT1 has been shown previously to maintain telomeres in mice). Lack of SIRT1 increases the amount of TERT remaining in the cytosol.
The upshot for us is that SIRT1 upregulation should accompany telomerase activators to increase the amount of telomerase that makes it into the nucleus and actually elongates telomeres. Personally, I intend combine my NMN/leucine/B6 protocol with my telomerase activation cycles, described as separate protocols here [3].
Edited by QuestforLife, 13 June 2024 - 08:35 AM.
#1003
Posted 13 June 2024 - 06:41 PM
Stunning confirmation of my theory that epitalon does not activate the telomerase gene
I’ve previously talked about:
-
How various methods of detecting telomerase are not the same and how immunochemistry is unreliable [1]; and
-
How I don’t believe epitalon activates telomerase but instead that it increases the assembly of telomerase in the nucleolus [2] and [3];
This recent paper [4] shows that the most commonly used antibody to detect telomerase is in fact detecting nucleolin, which is a protein involved in shepherding RNA into the nucleolus. Note that the antibody being discussed (NCL-hTERT) is EXACTLY the one I reference being used in [1] as overstating telomerase protein levels compared to other methods of measurement.
Immunodetection of human telomerase reverse-transcriptase (hTERT) re-appraised: nucleolin and telomerase cross pathsSurprisingly, mass spectrometry, two-dimensional gel analysis and immunofluorescent experiments revealed that the most frequently used hTERT immunoprobe, a mouse monoclonal antibody that was claimed to be directed against an hTERT protein epitope, in fact recognizes nucleolin rather than telomerase.
And get this…what antibody did Khavinson use in 2003 to ‘prove’ epitalon activated telomerase. I quote from his landmark paper [5]:
In order to detect telomerase protein catalytic subunit, fetal fibroblasts were put onto sterile slides…Immunohistochemical staining was carried out using murine monoclonal antibodies to human telomerase catalytic subunit NCL-hTERT.
To me, this is a stunning confirmation of my earlier theory and has practical implications in confirming that epitalon does not activate the telomerase gene, but instead increases its assembly into the active protein form.
I should mention here that Khavinson did also use a TRAP assay for telomerase activity (ability to elongate lab-made telomeres) and did find that epitalon elongated (lab-made) telomeres. Now, cells in vitro divide much more than in a human body, so they will have some telomerase gene expression simply because the gene cannot be repressed during S-phase. So, it is entirely plausible that epitalon maximises the small quantity of HTERT RNA gene expression into a respectable amount of telomere elongation via increasing HTERT location to the nucleolus using the nucleolin protein, which is what is staining Fig 1 so dark in the paper. Therefore people can use epitalon to get longer telomeres in their fast dividing cells, it is just not happening via activating the telomerase gene.
As I stated before [2], epitalon should be paired with a genuine telomerase activator for maximum effect. In addition, we now also know that SIRT1 is important in stabilising telomerase against ubiquitination (break down) and locating it to the nucleus where it can elongate telomeres, so we can now add sirtuin activators to our telomere stack.
I feel that we are now getting much closer to understanding the whole chain of events required to elongate telomeres:
-
HTERT gene is activated producing telomerase RNA (use telomerase activator)
-
HTERT RNA is moved into the nucleolus and assembled along with the TERC template into active telomerase (epitalon)
-
Telomerase is protected from breakdown and moved kept in the nucleus where it can add to telomeres (SIRT1 activator)
There are no doubt other stages such as the extent of TERC production and the availability of guanine for telomerase elongation, and others we haven’t yet worked out. But the picture is becoming clearer.
References
[4]Wu YL, Dudognon C, Nguyen E, Hillion J, Pendino F, Tarkanyi I, Aradi J, Lanotte M, Tong JH, Chen GQ, Ségal-Bendirdjian E. Immunodetection of human telomerase reverse-transcriptase (hTERT) re-appraised: nucleolin and telomerase cross paths. J Cell Sci. 2006 Jul 1;119(Pt 13):2797-806. doi: 10.1242/jcs.03001. Epub 2006 Jun 13. PMID: 16772337.
[5] Khavinson VKh, Bondarev IE, Butyugov AA. Epithalon peptide induces telomerase activity and telomere elongation in human somatic cells. Bull Exp Biol Med. 2003 Jun;135(6):590-2. doi: 10.1023/a:1025493705728. PMID: 12937682.
Edited by QuestforLife, 13 June 2024 - 06:44 PM.
#1004
Posted 13 June 2024 - 09:15 PM
I was probably overly harsh with the paper because of the way they presented things. I do agree it is an interesting result and stem cell exhaustion is clearly an important component of ageing, we can agree.
I am still not certain an aggressive senolytic protocol would be as beneficial for humans as mice; your aunt's story is interesting and very relevant: was the depletion of intestinal lining because stem cells were killed by chemo? Or was it that senescent cell numbers blocked the division of stem cells? Or did senescent cells drive excessive division of (stem) cells and exhaust them this way? Which of these mechanisms is dominant will decide what treatment course is preferable
a very big flaw in that experiment was not taking telomere length measurements, which they should have done considering CaG is a know telomerase activator. Without it is difficult to tell exactly how much it's senolytic properties were responsible for the deaging of mice versus it's telomerase activation properties.
#1005
Posted 06 July 2024 - 10:03 AM
TERT activation targets DNA methylation and multiple aging hallmarks
Many thanks to Chemically_Charmed for supplying the paper.
Background: This is a paper authored among others by Ron Depinho, famous years ago for this paper [1] where they induced (human-like) ageing in mice through telomerase deactivation and then reversed it by reactivating telomerase.
In the current paper [2] mice were genetically engineered to carry the HTERT gene plus its cis regulatory elements. This enabled researchers to see effects on this gene as it would occur in humans rather than upon the MTERT gene, which is generally active in most mice tissues but downregulated with age. Tests were carried out both on cells extracted from those mice (from the ears) and on the mice themselves.
Tests were also carried out in human cells: lung fibroblasts and Werner Syndrome fibroblasts. Presumably this allowed them to eliminate the possibility that the MTERT gene was also stimulated. I would have far preferred they used normal human cells passaged to near senescence rather than WS cells, but it is better than nothing.
Screening found a small molecule which upregulated the human telomerase gene (HTERT) in all tissues examined.
In a nutshell, what's good, what's bad?
Bad: TERT RNA upregulation was mild, maybe x2, if that
Good: TERT RNA upregulation occurred across all tissues tested
Bad: There is no comparison with HELA or any other TERT positive cell type for the extent of HTERT activation;
Bad: and there is no TRAP assay for telomerase activity; but
Good: They did show the molecule increased telomere length in human cells
Good: and reduced DNA damage foci due to short telomeres
Good: and increased proliferative ability in cells beginning to slow divisions
Bad: but that was in Werner Syndrome cells!
Good: TERT activation was VERY specific; only a couple of genes upregulated and translation in general not affected at all (means this may synergise with other approaches)
Good: The molecule has wide distribution in the body, including nervous system and brain; clearance took 3 hours (in mice)
Good: protein levels of TERT increased in PBMCs in proportion to increase in RNA levels, which is useful to know
Bad: but they looked at protein level using immunoblotting (not accurate)
Good: but they didn’t use the antibody NCL-HTERT known to be non specific to telomerase [3]
On to the effects…
Good: with only a week course of this molecule, senescence and inflammatory markers were reduced (more than halved) in old mice
Good: and this occurred across a wide range of tissues, not just blood cells
Good: and increased general markers of growth and natural killer cell activity
Good: and longer term treatment (6 months) in old mice reduced senescent cell burden
Bad: this was all injected 6mg/Kg/day
Good: The molecule had strong effects in the brain, increasing adult neurogenesis and decreasing microglia activation and inflammation. Aside: TERT seems to have particularly strong effects in the brain, as we've seen before with gotu kola extracts [4], and I speculate that this is due to high mitochondrial densities in those tissues.
Good: The molecular benefits in the brain also extended to actual benefits in old mice, including cognitive tests, balance and strength when injected with the substance 3 times/week.
Stepping back
Unlike with TAM818 no medicinal chemistry was done, they simply worked with the best molecule they found from screening. So, this molecule is unlikely to represent the best possible. Having said that, its molecular action was incredibly precise, with no side effects.
I am disappointed they didn't do more and better measurements of the extent of telomerase activation; I assume it was because the increase in levels were so mild. Nevertheless the benefits were real. Again, I speculate most of these effects were through non canonical mitochondrial benefits rather than through lengthening telomeres.
The study could be taken most usefully as a proof of the benefits of mild telomerase activation, rather than this being a super molecule, and secondarily that HTERT RNA upregulation does seem to feed through to actual telomerase production.
Other molecules like TAM818 do not have this level of evidence behind them, and other compounds like gotu kola extracts have a plethora of effects, some good, some bad. We do have evidence for the effects of TA-65 on mice, and they are not as promising as these results. So overall I'd say we have strong evidence for good effects with this molecule rather than weak evidence for very good effects (TAM818) or strong evidence for weak effects (TA-65). So, this paper is a step forward.
The big question is bioavailability. It is a small molecule and it gets everywhere once it is injected. Is it orally bioavailable? I suspect so because of its low molecular weight and the fact it is fat soluble.
References
[1] Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadiñanos J, Horner JW, Maratos-Flier E, Depinho RA. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011 Jan 6;469(7328):102-6. doi: 10.1038/nature09603. Epub 2010 Nov 28. PMID: 21113150; PMCID: PMC3057569.
[2] Shim HS, Iaconelli J, Shang X, Li J, Lan ZD, Jiang S, Nutsch K, Beyer BA, Lairson LL, Boutin AT, Bollong MJ, Schultz PG, DePinho RA. TERT activation targets DNA methylation and multiple aging hallmarks. Cell. 2024 Jun 21:S0092-8674(24)00592-0. doi: 10.1016/j.cell.2024.05.048. Epub ahead of print. PMID: 38908367.
[4] Tsoukalas D, Buga AM, Docea AO, Sarandi E, Mitrut R, Renieri E, Spandidos DA, Rogoveanu I, Cercelaru L, Niculescu M, Tsatsakis A, Calina D. Reversal of brain aging by targeting telomerase: A nutraceutical approach. Int J Mol Med. 2021 Nov;48(5):199. doi: 10.3892/ijmm.2021.5032. Epub 2021 Sep 13. PMID: 34515324; PMCID: PMC8448543.
Edited by QuestforLife, 06 July 2024 - 10:05 AM.
#1006
Posted 01 August 2024 - 06:31 AM
Since QuestForLife has discussed ROCK inhibitors at length in this thread, I thought I would link to a post I just made on the cardiac glycoside Ouabain, which shows very promising and interesting results for longevity, although it activates ROCK rather than inhibiting it. One study found that Ouabain had similar results to heterochronic parabiosis at a transcriptomic level. Ouabain lengthened fruit fly max lifespan by 25% when administered in very low doses. Ouabain is a prescription drug, but it is used only in low doses due to toxicity. Originally, Ouabain was used by African tribes to make poison arrows!
https://www.longecit...kes-the-poison/
#1007
Posted 18 August 2024 - 05:21 PM
Since QuestForLife has discussed ROCK inhibitors at length in this thread, I thought I would link to a post I just made on the cardiac glycoside Ouabain, which shows very promising and interesting results for longevity, although it activates ROCK rather than inhibiting it. One study found that Ouabain had similar results to heterochronic parabiosis at a transcriptomic level. Ouabain lengthened fruit fly max lifespan by 25% when administered in very low doses. Ouabain is a prescription drug, but it is used only in low doses due to toxicity. Originally, Ouabain was used by African tribes to make poison arrows!
I'll read the paper when I get chance. At first glance it seems somewhat unbelievable that one drug would have so many effects - particularly when those effects are just what will get you published in ageing research (senolytic, parabiosis, etc) - but I'll suspend judgement for now!
#1008
Posted 21 August 2024 - 10:19 PM
Introduction: Functional reduction of telomeres can induce DNA damage response through cell cycle checkpoints and contribute to the senescence of stem cells. The effect of exosomes on the aging and rejuvenation of hematopoietic stem cells (HSCs) is not well known. Therefore, the present study is designed to examine the impact of plasma exosomes derived from young and old individuals on hTERT and P16 expression involved in the cellular aging process.
Methods: Exosomes isolated from four young (Y-Exo) and four old (O-Exo) men were evaluated for CD63 protein expression, morphology, size and zeta potential. HSCs were treated with exosomes, and then, the cell viability and the mRNA expression (hTERT and P16) were evaluated using MTT and qRT-PCR methods, respectively. To measure the hTERT protein level, a western blot technique was performed.
Results: The gene expression of hTERT was significantly decreased in HSCs treated with 5 μg/ml (O5-Exo) and 10 μg/ml (O10-Exo) doses of exosomes obtained from elderly individuals compared to the cells treated with young exosomes and the untreated HSCs (p < 0.05). In addition, there was a profound elevation of hTERT protein in the HSCs treated with both doses of young exosomes in comparison with the cells treated with both doses of old exosomes (p < 0.05). Moreover, P16 expression was markedly upregulated in the O5-Exo and O10-Exo groups compared to the untreated group (p < 0.05).
Conclusion: Our findings reinforce the concept that depending on the age of individuals, circulating exosomes may acquire properties that affect the pathways involved in the aging process in HSCs.
#1009
Posted 22 August 2024 - 08:50 AM
A preprint of a paper claims that exosomes isolated from the plasma of young men increased the expression of hTERT in hematopoietic stem cells in vitro."The Effect of Circulating Exosomes Obtained from Young and Old Individuals on the Aging related hTERT and P16 Expression in Hematopoietic Stem Cells"
I was genuinely confused when you posted this that you didn't refer to my earlier post on the subject, and then on checking, noticed I never posted it!
I wrote this a month ago, based on a different paper, which I think is a little more interesting than the one you post, for reasons you'll see.
The interesting idea of exosomes full of telomerase to treat ageing
We have discussed cells lending each other telomeres before (1), but here the intriguing possibility is raised of using exosomes from HTERT immortalised cells (2) to supply telomerase to ageing cells (or, by implication, an ageing body).
I have no idea how practical this would be: immortalise human cells, passage them to great number, then separate the exosomes by centrifugation …But given the enormous expense of HTERT gene therapy - thus far the only way of supplying enough telomerase to actually lengthen telomeres - this method deserves mention.
Quickly, what are the main flaws of the paper:
-
HTERT is reported as a fold change: but how can you measure the amount of HTERT RNA expression in telomerase negative cells? This is dodgy, in my view, and means we can only tell that yes, HTERT expression increased…by some amount.
-
Telomerase protein levels were also reported: but as we know, protein levels of telomerase are unreliable because immunohistochemistry is unreliable (particularly for telomerase).
-
No TRAP assay for telomerase activity done.
-
We don’t know if all exosomes have telomerase in them, or just some - but the paper claims the exosomes were homogeneous (how you tell this, I don't know)
-
Only one cell type was examined
-
This was all in vitro, we don’t know where these exosomes would go in an actual body, or what parts they would reach.
But, in its favour:
-
They did show actual telomere lengthening in the recipient telomerase negative human cells;
-
The did show senescing telomerase negative human cells were again able to divide (at least a couple more times; the experiment was not continued long enough to see how durable this rejuvenation was) ;
-
They did show senescent markers in the recipient telomerase negative human cell were decreased;
by treatment.
Bottom line
This is a promising new avenue in telomerase treatment research. My preference would be for an effective (and cheap) small molecule, but exosomes are relatively accessible and can be supplied by some doctors, so this seems like a possibility for the near term - in contrast to gene therapy that seems well out of reach for the foreseeable.
[2] Liu Y, Sheng Z, Sun L. Exosomes derived from hTERT-immortalized cells delay cellular senescence of human fibroblasts. Exp Gerontol. 2024 Jul 8:112508. doi: 10.1016/j.exger.2024.112508. Epub ahead of print. PMID: 38986855.
ps things that have just occurred to me:
We shouldn't underestimate the cost of immortalising cells and growing them to a number large enough to harvest significant exosomes, but I expect it would still be cheaper than gene therapy.
Also, how good are we are detected exosomes as opposed to very small cells - not sure.
Finally, this is a nice confirmation of the conditional reprogramming concept discussed previously, when senescent embryonic cells are used to supply the telomerase and ROCK inhibitors to de-differentiate terminally differentiated cells [3]. We never knew how the telomerase got from the feeder cells to the cells being passaged: I was always confused by how we are told telomerase is too big to enter cells, and yet it clearly does (which to my mind meant that direct telomerase protein treatment is a viable option: this was discussed somewhere back in this thread). Well now we know how the telomerase got into the cells: exosomes!
[3] https://www.longecit...es/#entry864534
Edited by QuestforLife, 22 August 2024 - 08:51 AM.
#1010
Posted 23 August 2024 - 04:11 AM
Very interesting Quest! Thanks for sharing. It is hard to beat you to the punch on telomere research. That is a very interesting idea you have put forward - to create exosomes from HTERT immortalized cells. In addition to being cheaper than HTERT gene therapy, this approach would also be more natural and likely safer . In the meantime, the next best thing may be exosomes from umbilical cord plasma.
You have written before that increasing cellular NAD levels should be effective in greatly reducing telomere shortening, and that this reduction in shortening should appear in conventional leukocyte telomere tests as an increase in telomere length. I am happy to report that for the past 5 months I have been taking a liposomal blend of NAD precursors and that I appear to have greatly reduced my telomere shortening. I did testing with TruDiagnostic at baseline a year ago, then 5 months ago, and then again just last month. See here for my new write up on my significant 1 year results: https://www.longecit...ge/#entry932200
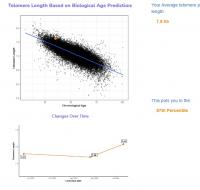
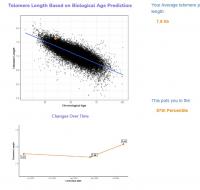
My epigenetic estimate of my telomere length increased to 7.63 Kb (equivalent to someone 18.4 years old). My baseline was 7.51 Kb (equivalent to someone 26 years old). Notably, my telomere length only increased after I doubled my NAD precursor dosage. My telomere length had not meaningfully changed at my half year test. I know that you are not a fan of this epigenetic estimate of telomere length, but since this result is so dramatic hopefully it suggests that my telomere shortening has been greatly slowed.
Edited by dlewis1453, 23 August 2024 - 04:13 AM.
#1011
Posted 29 August 2024 - 04:55 AM
Reporting on the Mitotic Clock, my results with this clock, and its relationship with epigenetic proxies of telomere length
Introduction: TruDiagnostic has released a new test that the readers of this thread might find interesting - the Mitotic Clock, an estimator of cellular turnover, and by extension stem cell turnover1. Maybe we can uncover some insights by comparing my results on the Mitotic Clock over the past 12 months with my results on the epigenetic proxy of telomere length over the past 12 months.
Mitotic Clock: The Mitotic Clock is calculated by looking at 385 locations (PCGT/PRC2-marked promoter CpGs) that are unmethylated at birth, but gain DNAm as chronological age increases.2 The algorithm to sort through this data was trained on a large cohort of healthy individuals, as assessed in one tissue type (blood).This epigenetic clock is meant to estimate the amount of stem cell division in tissue samples and to estimate cancer risk. The mitotic clock exhibits acceleration in normal buccal tissue from smokers compared with nonsmokers, and in normal breast tissue from patients with cancer compared with healthy women, making it a unique biological clock for estimating cancer risk.
In 2016, a paper tried to explain why cancer risk is so different in some tissues than others. The research done by a group at Johns Hopkins (Tomasetti, Et. al)3, showed that the lifetime risk of cancers of many different types is strongly correlated (r2=0.81) with the total number of divisions of the normal self-renewing cells maintaining that tissue’s homeostasis.
Results: QuestForLife has previously criticized the epigenetic proxy for telomere length on the grounds that:
The case in point is the leukocyte methylation telomere clock, which doesn't measure telomeres at all! It advances with cell divisions regardless of the telomere length, so we don't know if you did stop telomere shortening if the clock would continue to have predictive power for health outcomes or not.
Now that I have epigenetic measures of both cellular division and telomere length, I thought it would be interesting to share my results here and see if any insights can be gleaned from them.
I have reattached my results for my epigenetic proxy of telomere length (1st attachment). I have also attached screenshots of my results on the Mitotic Clock. Note that my three results on the Mitotic Clock come from the same samples as my three telomere proxy results, so we can make direct comparisons between the two tests.
1. Baseline Test: What stands out to me is that at baseline, before starting my protocol, my epigenetic proxy of telomere length was long (corresponding to an age of 26 vs my chrono age of 34 at the time). Likewise, my cellular division rates as measured by the Mitotic Clock were quite low.
2. Halfway Point: Then at the halfway point my telomere length estimate declined slightly, while my cellular division rate increased considerably to an average level. Note that this test came right after a period where I was sick back to back with two strong viral infections. Shortly after this test, I doubled my dosage of liposomal NAD precursors, because a cellular NAD test indicated that my previous dosage was not having an impact. (Independent lab testing confirmed that the supplement brand I was using was properly dosing their products, so quality control was not the issue)
3. Then at the 12 month point point, my estimate of telomere length shot upwards to the levels of an 18 year old, and my cellular division rates dropped back down to a level even lower than they had been at baseline.
I should also mentioned that during this 12 month period I experienced significant reductions in epigenetic age as measured by the OmicAge and SymphonyAge clocks. See here for more on this: https://www.longecit...ge/#entry932200.
1 "A new timepiece: an epigenetic mitotic clock" https://genomebiolog...3059-016-1085-y
2 For background, mitosis is the process by which a cell replicates its chromosomes and then segregates them, producing two identical nuclei in preparation for cell division. Mitosis is generally followed by equal division of the cell's content into two daughter cells that have identical genomes.
3 :text=Why%20do%20some%20tissues%20give,number%20of%20stem%20cell%20divisions.
Edited by dlewis1453, 29 August 2024 - 04:59 AM.
#1012
Posted 29 August 2024 - 09:30 AM
Reporting on the Mitotic Clock, my results with this clock, and its relationship with epigenetic proxies of telomere length
Both of these are mitotic clocks, one is just the inverse of the other. Neither measure telomere length.
Is your protocol increasing telomerase expression or telomere assembly?
I have speculated that increasing NAD+ might decrease telomere loss/division as has been seen in vitro, or it might - as I have recently written - increase telomerase import to the nucleus via an increase in sirtuin 1, thereby increasing telomerase activity at the telomere.
But it might simply be that higher NAD+ is signaling less energy, so decreasing cellular mitosis as a result. Under this scenario, your improvements (in both clocks) are simply signaling that you have decreased the drawdown of blood cells from the stem cell compartment. Even if you had results from a genuine telomere measurement and it showed an increase in telomere length, that could also be the result of decreased division, I.e. the progenitor cells ‘recovering’ telomere length because they are being replaced from the stem cell compartment faster than they are being made to differentiate and divide by downstream cell requirements.
What we'd need is a genuine telomere test that showed increased length at the same time a mitotic clock showed no decrease in the rate of division (necessitating 2 lots of tests to see the changes). This would mean the body is continuing to supply cells at the proper rate (outside of illness) but also managing to increase telomere length.
#1013
Posted 30 August 2024 - 06:03 AM
I have been taking liposomal formulations of the following supplements for the past year:
1. Ca-AKG
2. Spermidine
3. EGCG
4. Vitamin C
5. NAD precursor blend (NR, NMN, & NAD)
I recently added in HMB/Leucine, Lipo-Trans Resveratrol, and Vitamin B6 to copy your newest protocol, but this addition is not reflected in my last test.
Of those five supplements, the only one that I would expect to affect telomeres would be the NAD precursors and NAD itself.
_____________________________________________________________
But it might simply be that higher NAD+ is signaling less energy, so decreasing cellular mitosis as a result. Under this scenario, your improvements (in both clocks) are simply signaling that you have decreased the drawdown of blood cells from the stem cell compartment. Even if you had results from a genuine telomere measurement and it showed an increase in telomere length, that could also be the result of decreased division, I.e. the progenitor cells ‘recovering’ telomere length because they are being replaced from the stem cell compartment faster than they are being made to differentiate and divide by downstream cell requirements.
Even if all that is happening is a decrease in cellular mitosis, wouldn't this be expected to increase lifespan as well? Decreasing drawdown from the stem cell compartment should delay aging of the stem cell compartment.
_____________________________________________________________
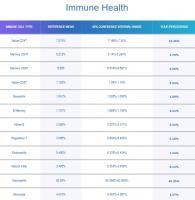
While we are on the topic of cellular division, how do you reconcile my decrease in cellular division with my significant increase in naive T cells and naive B cells? (see attachment for my latest immune cell results). Trudiagnostic estimated my immune age (calculated from immune cell ratios) as being 18 years old. I wonder if I am experiencing some thymic regeneration. My theory is that my supplements are putting me into a metabolic state similar to calorie restriction, and a study in 2022 found that calorie restriction in humans for 2 years enabled increased T cell production and increased thymic mass.
Compared with baseline, sustained CR for 2 years significantly (P < 0.05) increased thymic mass (Fig. 1B) as well as total thymic volume in study participants.
https://www.ncbi.nlm...es/PMC10061495/
_____________________________________________________________
Lastly, you wrote...
What we'd need is a genuine telomere test that showed increased length at the same time a mitotic clock showed no decrease in the rate of division (necessitating 2 lots of tests to see the changes). This would mean the body is continuing to supply cells at the proper rate (outside of illness) but also managing to increase telomere length.
I want to do this experiment for you. It would be very interesting to see the results. Is there a telomere test in the U.S. that you recommend?
Edited by dlewis1453, 30 August 2024 - 06:04 AM.
#1014
Posted 31 August 2024 - 08:50 PM
Yes it would be beneficial, supposing that we can reduce the draw down without reducing immunity, regeneration, virility, quality of life, etc. Remember the aim of life extension is not to put your body in middle aged economy mode early! Having said that, NAD+ precursors tend to give one more energy, atleast in the short term.Even if all that is happening is a decrease in cellular mitosis, wouldn't this be expected to increase lifespan as well? Decreasing drawdown from the stem cell compartment should delay aging of the stem cell compartment.
You answered it yourself. Mild CR (and perhaps it's mimetics) seems to rebalance the immune system. It's no surprise though, is it? Decreased division, and very likely, differentiation, will balance back to naive cell types, and would also be expected to reduce epigenetic age, which likely is measuring the ratio of different cell types rather than changes within individual cells._____________________________________________________________
While we are on the topic of cellular division, how do you reconcile my decrease in cellular division with my significant increase in naive T cells and naive B cells? (see attachment for my latest immune cell results). Trudiagnostic estimated my immune age (calculated from immune cell ratios) as being 18 years old. I wonder if I am experiencing some thymic regeneration. My theory is that my supplements are putting me into a metabolic state similar to calorie restriction, and a study in 2022 found that calorie restriction in humans for 2 years enabled increased T cell production and increased thymic mass.
_
I used Lifelength when it used to be available in the UK. But in the US there probably are lots of options. Won't be cheap though. Telomere tests, done properly, aren't so simple as looking at methylation.____________________________________________________________
Lastly, you wrote...
I want to do this experiment for you. It would be very interesting to see the results. Is there a telomere test in the U.S. that you recommend?
Edited by QuestforLife, 31 August 2024 - 08:52 PM.
#1015
Posted 03 September 2024 - 05:06 AM
Decreased division, and very likely, differentiation, will balance back to naive cell types, and would also be expected to reduce epigenetic age, which likely is measuring the ratio of different cell types rather than changes within individual cells.
The concern you raised above was certainly an issue in first generation epigenetic clocks. However, it seems that second and third generation epigenetic clocks are accounting for this. In their report on immune cells, TruDiagnostic states that they attempt to control for variations in different immune cell types when performing their epigenetic tests on blood samples. See below for a quote from their immune cell report.
As we age, we have overall fewer Naïve T Cells, Natural Killer Cells, Macrophages, Dendritic Cells, and other immune cell types throughout our body. However, concentrations of immune cell types also change based on what kind of sample or tissue you are analyzing, such as blood or saliva, regardless of age.
Each immune cell type, and its respective concentration, can indicate vastly different aging implications from other types of immune cells when isolated; forcing algorithm developers to ask, 'Is this pattern actually caused by aging, or is this pattern caused by the type of cell and the type of tissue we are examining?'Further potential for data pollution rests in whether or not someone's immune system was actively or recently fighting an illness at the time of sample collection; which can cause temporary changes in immune cell concentrations.
Say we were to isolate Naïve CD8 immune cells from Memory CD8 immune cells (both of which are found in different concentrations in your blood sample) to determine your biological age based on those cells alone. The Naïve CD8 cells might say you're 40 years old, for example, while the Memory CD8 cells would say you're 55 to 60 years old.Instead of addressing this challenge by completely excluding immune biomarkers, all of our algorithms were trained and developed with a weighted and controlled representation of each immune cell type and its concentration in blood tissue.
TruDiagnostic is also working on immune cell deconvolution in saliva samples, see below.
“Almost all epigenetic algorithms have been created via blood cell datasets, which is why blood tissue is widely used for accurate methylation detection and quantification. However, even in blood, measurements can be highly variable if the different types of immune cell subsets are not controlled for during the analysis. This is where cell deconvolution methods are so vital, as they allow us to control for these variables during analysis.” Says Dr. Dwaraka, Head of Bioinformatics at TruDiagnostic.
Saliva epigenetic samples face the same problem but face more confounding variables due to greater number of epithelial cell types found from the buccal tissue. To alleviate this issue, we have created this saliva deconvolution method to make sure we accurately control for these cell quantities which can change with age and conditions like smoking. This is a critical step in making sure that the innovations created from blood methylation can also be applied to Saliva tissue samples.
https://www.trudiagn...1SthY9frt3zWNEX
Despite their broad use across both research and commercial settings, DNAm clocks have notable limitations. One such limitation is the difficulty of accurately reducing dimensionality due to the technical noise in measuring individual CpGs, which subsequently affects the precision 22. The heterogeneity of immune cell subsets is also a confounder of DNAm aging estimates, and whilst cellular deconvolution methods have been applied, to date immune deconvolution has considered a limited number of cell types23,24. Further, the inclusion of a CpG in a predictive aging clock does not necessarily imply causality nor functionality25, and identifying causal CpGs from DNAm clocks remains a challenging task, one that limits their biological potential.
.........
OMICmAge was generated via a penalized elastic net regression model of EMRAge that included methylation CpG values, relative percentages of 12 immune cell subsets, 396 EBPs (Extended Table S2), age and sex as features in the model.
Whether the methods being used here to control for variations in cell populations are sufficient, I do not know. TruDiagnostic and epigenetic researchers have at least partially accounted for this issue though.
#1016
Posted 04 September 2024 - 03:27 PM
As to their comments on distinguishing between causal and methylation changes downstream of ageing, this is quite funny. It is incredibly complicated but very likely none of these changes are causal. The cells are just adjusting to the environment they are in (the body).
I realise this is a statement that will surprise many. But as people who are willing to invest money into testing as a way of guiding health changes, we should consider the possibility that methylation patterns reflect ageing, but do not drive it. This may seem like a minor point, and that these clocks are useful even in this case. But not necessarily. If they reflect ageing but do not drive it, then if we make changes to revert methylation to a younger state, we may be harming ourselves. This is because if those methyaltion changes are adaptations the best way to get them to change back is to adjust the environment , but if we force them back by some other means, we will be causing the body to operate in a less optimal state. This is the elephant in the room that no one in this new industry wants to talk about.
You mentioned my glycine paper [1]. Well SHMT2 downregulation was claimed to be causing ageing (via respiratory defects in mitochondria), and yet I've shown quite convincingly this is just a cellular response to a glycine shortage in the body, and actually keeps the cells going longer.
Now some epigenetic changes might be causal. Who knows! Certainly lots of them will LOOK causal. But i'm guessing they'll all turn out to be adaptations. So we should be careful in believing the clever and superficially convincing pronouncements of the developers of nth generation epigenetic clocks. As already stated we might even get worse health outcomes by moving in the ‘right’ direction. For example, people with more SHMT2 expression are generally younger. But if we were to force an old person's cells to express more SHMT2 like a young person (without supplying more glycine) there would be advantages and disadvantages, but overall I would be willing to bet that the changes would be for the worse.
As a precaution, make sure you monitor your health in other ways (which I'm sure you are already doing), and do not only rely on ageing clocks, particularly methylation ones.
[1] https://www.academia...uction_with_age
#1017
Posted 20 September 2024 - 08:23 AM
Nicotine is Amazing! [1]
Nicotine appears to be a strong candidate to add to my NAD+/sirtuin stack (currently Leucine/B6/NMN and low dose Resveratrol + Cu), and through various posited mechanisms such as ROS control and sirtuin shepherding of telomerase into the nucleus, my telomerase stack as well (currently TAM818 with less regular use of epitalon and gotu kola).
Note these stacks have been merged since June and are taken 3 days/week [2].
Highlights from the 2023 paper:
-
Nicotine increases NAMPT (the pathway that recycles NAM into NAD+) across many tissues (cortex, hippocampus, heart, liver, muscle and kidneys)
-
Nicotine increases SIRT1 binding of NAMPT across all those same tissues examined, often to greater than youthful levels
-
and subsequently SIRT1 deAcetylates NAMPT across all tissues examined, albeit some more than others
-
These effects were evident at nanogram levels and independent of nicotine's known acetylcholine effects
-
Nicotine regulates metabolism both at the cellular and whole body (mouse) level: increased dependence on glucose metabolism with age was mitigated through nicotine treatment (as measured by JAK2 and STAT3)
-
Other benefits to mice included increasing neurogenesis, inhibiting neuroinflammation and these benefits did translate to improved function in cognitive tests like the water maze
-
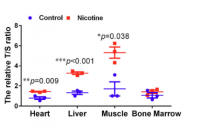
Most interestingly for this thread, the benefits appears to be underpinned by an increase in antioxidant activity (as measured by TEAC and SOD activity) and an increase in telomere length
-
Telomere length (as measured by the T/S ratio) increased both in nicotine treated cell culture and also (and more relevantly) in tissues taken from aged mice, compared to mice of the same age who were not treated (over a 6 month period).
-
Increases were significantly statistically, and also significant in absolute magnitude, ranging from x1.5 to x5
-
The only tissue examined that did not seem affected was bone marrow, which suggests the benefits are most likely through non-telomerase based mechanisms, i.e. decreasing telomere loss rather than increasing it through increased telomere generation* (though this is speculative on my part)
I attach figure 10e.
Edit
There is also useful information in the supplementary material [3]
- SIRT1 activity is not increased by nicotine directly (only its association with NAMPT) suggesting potential synergy with the rest of my stack (that increases NAD+ and SIRT interaction with NAD+)
- Nicotine adjusted the telomerase associated shelterin protein levels to increase protection of the telomere* supporting my earlier speculation nicotine's benefits are not directly from telomerase
- Although a full lifespan study was not carried out, nicotine treatment from 12-18 months of age did seem to increase survival at 18 months from 30 to 50% (admittedly in a small group). Note these were all male mice.
References
[1] Yang L, Shen J, Liu C, Kuang Z, Tang Y, Qian Z, Guan M, Yang Y, Zhan Y, Li N, Li X. Nicotine rebalances NAD+ homeostasis and improves aging-related symptoms in male mice by enhancing NAMPT activity.
Nat Commun. 2023 Feb 17;14(1):900. doi: 10.1038/s41467-023-36543-8. PMID: 36797299; PMCID: PMC9935903.
[2] https://www.longecit...-34#entry931383
[3] https://static-conte..._MOESM1_ESM.pdf
Edited by QuestforLife, 20 September 2024 - 08:38 AM.
#1018
Posted 03 November 2024 - 03:34 PM
#1019
Posted 03 November 2024 - 07:56 PM
Nicotine is Amazing! [1]
.....
If taking nicotine in supplemental form -say patches, any idea if the standard dosed patches are sufficient to benefit from these benefits? Or are these more "proof of concept" type conclusions which may not manifest at doses we can realistically take?
(talking about 20 mg nicotine patch for example)
#1020
Posted 04 November 2024 - 07:14 PM
If taking nicotine in supplemental form -say patches, any idea if the standard dosed patches are sufficient to benefit from these benefits? Or are these more "proof of concept" type conclusions which may not manifest at doses we can realistically take?
(talking about 20 mg nicotine patch for example)
On the contrary, the NAMPT benefits are achieved at nanogram nicotine levels and independent of its known effects on the nicotine acetylcholine receptor. So, you'd want a much lower dose than 20mg, I expect.
Also tagged with one or more of these keywords: telomeres, nad, nampt, ampk, resveratrol, allicin, methylene blue, nmn, sirtuins, statin
Science & Health →
AgingResearch →
Telomeres →
TELOM-R CITO DVR Pharm ( Telomeres in cancer treatment)Started by John Katz , 16 Apr 2025 |
|

|
||
Science & Health →
AgingResearch →
Telomeres →
Astragalus Influences Gut Stem Cells for Safe and Natural Telomerase ActivationStarted by osris , 12 Apr 2025 |
|

|
||
Science & Health →
Supplements →
Regimens →
Does Methylene Blue Impact Lifespan?Started by Michael Lustgarten , 08 Apr 2025 |
|

|
||
Science & Health →
Supplements →
Regimens →
My Current StackStarted by Rocket , 31 Dec 2024 |
|

|
||
Science & Health →
Supplements →
Boron (Boric Acid): Anti AGE, Gut Ribose production, etc?Started by Logic , 31 Dec 2024 |
|

|
2 user(s) are reading this topic
0 members, 2 guests, 0 anonymous users