.
F U L L T E X T S O U R C E : nature
To fight cancer and ageing, biologists are looking at how cells evict, kill or cannibalize less-fit rivals.
Yasuyaki Fujita has seen first-hand what happens when cells stop being polite and start getting real. He caught a glimpse of this harsh microscopic world when he switched on a cancer-causing gene called Ras in a few kidney cells in a dish. He expected to see the cancerous cells expanding and forming the beginnings of tumours among their neighbours. Instead, the neat, orderly neighbours armed themselves with filament proteins and started “poking, poking, poking”, says Fujita, a cancer biologist at Hokkaido University in Sapporo, Japan. “The transformed cells were eliminated from the society of normal cells,” he says, literally pushed out by the cells next door.
In the past two decades, an explosion of similar discoveries has revealed squabbles, fights and all-out wars playing out on the cellular level. Known as cell competition, it works a bit like natural selection between species, in that fitter cells win out over their less-fit neighbours. The phenomenon can act as quality control during an organism’s development, as a defence against precancerous cells and as a key part of maintaining organs such as the skin, intestine and heart. Cells use a variety of ways to eliminate their rivals, from kicking them out of a tissue to inducing cell suicide or even engulfing them and cannibalizing their components. The observations reveal that the development and maintenance of tissues are much more chaotic processes than previously thought. “This is a radical departure from development as a preprogrammed set of rules that run like clockwork,” says Thomas Zwaka, a stem-cell biologist at the Icahn School of Medicine at Mount Sinai in New York City.
But questions abound as to how individual cells recognize and act on weaknesses in their neighbours. Labs have been diligently hunting for — and squabbling over — the potential markers for fitness and how they trigger competitive behaviours. These mechanisms could allow scientists to rein in the process or to help it along, which might lead to better methods for fighting cancer and combating disease and ageing using regenerative medicine.
“Cell competition is on the global scientific map,” says Eugenia Piddini, a cell biologist at the University of Bristol, UK, who likens the buzz around this idea to the excitement that helped propel modern cancer immunotherapies. The better scientists understand competition, she says, the more likely it is that they will be able to use it therapeutically.
History repeats
During a blizzard that dumped more than 30 centimetres of snow this past February, biologists from about a dozen disciplines convened at a hotel at Lake Tahoe, California, for the first major meeting devoted to cell competition.
“It was a zoo of researchers,” says co-organizer Zwaka, and included biologists who study flatworms that can regenerate their whole body from a single cell, geneticists attempting to make interspecies chimaeras of mouse, monkey and rabbit embryos, and a keynote speaker who spoke about the terrible battles and cooperative campaigns waged in bacterial communities.
The snowbound attendees, about 150 in all, debated how and why cells size up their competition. And they celebrated the discovery that gave birth to the field.
In 1973, two PhD students, Ginés Morata and Pedro Ripoll were perfecting a way to track the various cell populations in a fruit-fly larva that would eventually develop into a wing. Working at the Spanish National Research Council’s Biological Research Center in Madrid, they introduced a mutation called Minute into a few select cells in the larva and left the rest of the cells unaltered.
Knowing that Minute cells grow slower than their unaltered neighbours, the scientists expected to find some smaller cells amid the wild-type counterparts. “Instead, we found that the cells disappeared,” says Morata, now a developmental biologist at the Autonomous University of Madrid in Spain.
On their own, Minute cells can develop into a fly that is normal — except for the short, thin bristles on their bodies that give the mutation its name. But when mixed with wild-type cells in the larva, the cells simply vanished. “Minute cells were not able to compete with the more vigorous, metabolically active wild-type cells,” says Morata. They described the activity as cell competition1. “It was a very surprising and interesting observation,” Morata says. But lacking the molecular tools to follow cell fates more closely, he and his colleagues let the finding simmer.
Twenty-six years later, postdocs Laura Johnston and Peter Gallant observed nearly the same phenomenon. Working with Bruce Edgar and Robert Eisenman, respectively, at the Fred Hutchinson Cancer Center in Seattle, Washington, they were studying a mutation in another fly gene, Drosophila Myc (dMyc), that also slows cell growth2.
“There was a eureka moment when Peter and I realized that these dMyc mutant cells would disappear,” says Johnston, now a developmental biologist at Columbia University Medical Center in New York City. They eventually showed that the mutant cells were forced to initiate a form of programmed cell death called apoptosis. “It was very clear that this was a competitive situation,” Johnston says.
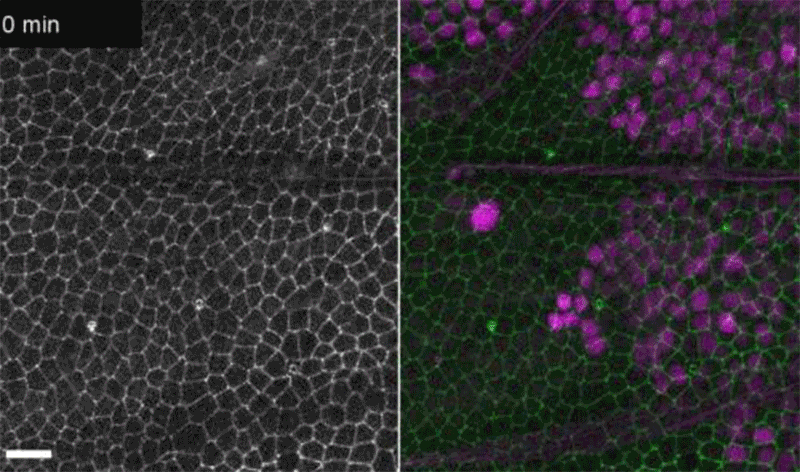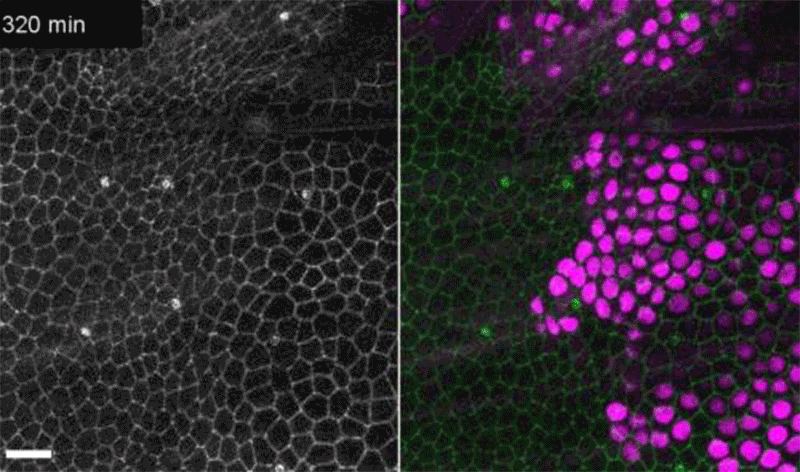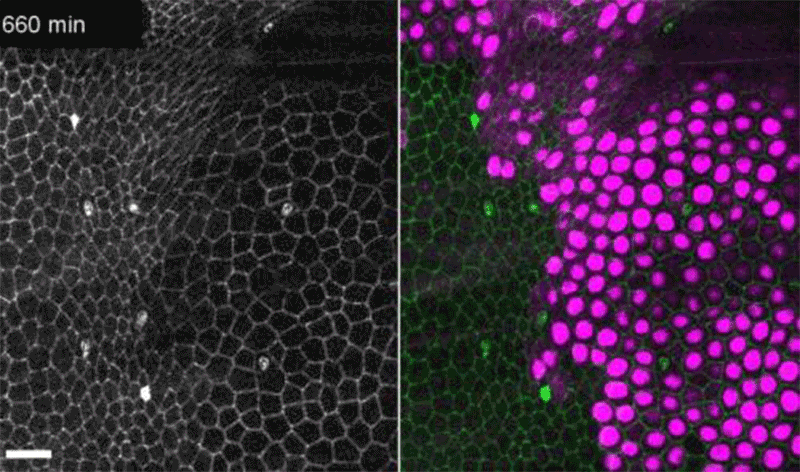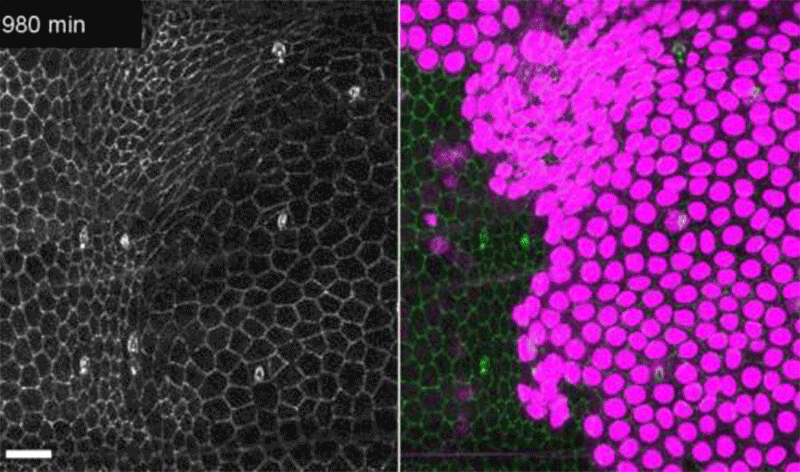
Fruit-fly cells with the oncogene Ras activated (purple) can outcompete neighbouring wild-type cells (green).Credit: Eduardo Moreno
Their 1999 paper ignited interest among scientists, including Morata. He jumped back into the fray with Eduardo Moreno, and they took advantage of modern molecular tools to repeat the Minute experiments. “The field blossomed from there,” says Johnston.
Myc acts as a master controller of cell growth, and Minute encodes a key component needed for synthesizing proteins — so it’s not surprising that reduced expression of those proteins makes cells less fit. But Johnston’s next finding took people by surprise. She showed that cells with an extra copy of normal dMyc outcompeted wild-type cells3. These fitter-than-wild-type cells came to be called “supercompetitors”.
Johnston’s discovery of supercompetition emphasized that cell competition is about the relative fitness of a group of cells, says Zwaka. If one cell is falling behind, the entire group of neighbours could decide it has to go. But on the flipside, they can also sense that certain cells are better and should survive.
Cell competition wasn’t simply about getting rid of defects; it was about survival of the fittest, with the less-fit ‘loser’ cells dying and the ‘winners’ proliferating. Importantly, competition was seen only when there was a mixture of genetically different cells, a phenomenon known as mosaicism. In this way, cell competition acts like a quality-control system, booting out undesirable cells during development.
Vying for viability
Fujita’s observation of the kicked-out kidney cells was one of the first hints that mammalian cells compete, too4. Soon after that work was published, researchers started to observe competition forcing out mutated cells from various other tissue types such as skin, muscle and gut.
The next most obvious place to look for competing cells was the mammalian embryo. In 2013, Zwaka’s team, and two other laboratories, probed mouse embryos at the earliest stage of development — those that have progressed just beyond a ball of cells. Zwaka’s group made mouse embryonic stem cells (ESCs) with a supercompetitor mutation that lowered expression of p53, an important quality-control protein that normally puts the brakes on cell division. When these cells were put into a mouse embryo, they quickly took over and developed into a normal mouse5. Similarly, Miguel Torres’s lab at the National Center for Cardiovascular Research in Madrid showed that supercompetition could be induced in an early mouse embryo using slight overexpression of the mouse Myc gene.
By artificially creating losers or winners, researchers could force cell competition into play. But Torres’s team, led by then-postdoc Cristina Clavería, also made the striking observation that Myc expression varied naturally in mouse ESCs. Cells in the embryo with approximately half the amount of the protein compared with their neighbours were dying by apoptosis. This was one of the first studies that strongly pointed to naturally arising cell competition6.
.../...
.














































