.
COMPLETE TITLE: Honokiol antagonizes doxorubicin‑induced cardiomyocyte senescence by inhibiting TXNIP‑mediated NLRP3 inflammasome activation
F U L L T E X T S O U R C E : International Journal of Molecular Medicine
Abstract
Senescence of cardiomyocytes is considered a key factor for the occurrence of doxorubicin (Dox)‑associated cardiomyopathy. The NOD‑like receptor family pyrin domain‑containing 3 (NLRP3) inflammasome is reported to be involved in the process of cellular senescence. Furthermore, thioredoxin‑interactive protein (TXNIP) is required for NLRP3 inflammasome activation and is considered to be a key component in the regulation of the pathogenesis of senescence. Studies have demonstrated that pretreatment with honokiol (Hnk) can alleviate Dox‑induced cardiotoxicity. However, the impact of Hnk on cardiomyocyte senescence elicited by Dox and the underlying mechanisms remain unclear. The present study demonstrated that Hnk was able to prevent Dox‑induced senescence of H9c2 cardiomyocytes, indicated by decreased senescence‑associated β‑galactosidase (SA‑β‑gal) staining, as well as decreased expression of p16INK4A and p21. Hnk also inhibited TXNIP expression and NLRP3 inflammasome activation in Dox‑stimulated H9c2 cardiomyocytes. When TXNIP expression was enforced by adenovirus‑mediated gene overexpression, the NLRP3 inflammasome was activated, which led to inhibition of the anti‑inflammation and anti‑senescence effects of Hnk on H9c2 cardiomyocytes under Dox treatment. Furthermore, adenovirus‑mediated TXNIP‑silencing inhibited the NLRP3 inflammasome. Consistently, TXNIP knockdown enhanced the anti‑inflammation and anti‑senescence effects of Hnk on H9c2 cardiomyocytes under Dox stimulation. In summary, Hnk was found to be effective in protecting cardiomyocytes against Dox‑stimulated senescence. This protective effect was mediated via the inhibition of TXNIP expression and the subsequent suppression of the NLRP3 inflammasome. These results demonstrated that Hnk may be of value as a cardioprotective drug by inhibiting cardiomyocyte senescence.
Introduction
Doxorubicin (Dox) is an anthracycline, which are effective anticancer drugs widely used in clinical practice (1). Despite the effectiveness of Dox against cancer, it is associated with severe dose-limiting cardiotoxicity, which typically manifests as subclinical cardiac dysfunction or clinical heart failure at the end of chemotherapy or after several years (2). This late-onset form of anthracycline cardiotoxicity is referred to as chronic and has a complex pathogenesis (3,4). Cardiomyocyte senescence has been previously suggested as a mechanism underlying Dox-induced cardiotoxicity (5), which can, at least partly, result in cardiac remodeling and dysfunction (6). Therefore, senescence is pivotal to the progression of delayed anthracy-cline cardiomyopathy in mice (7), as well as in humans (8). Accordingly, regulation of cardiomyocyte senescence may promote the development of cardioprotective strategies and reduce the incidence and mortality of cardiovascular disease in the early and late stages of cancer treatment (9). However, the clinical effectiveness of anti-senescence therapies remains poor. Therefore, investigating novel therapeutic strategies to alleviate Dox-induced cardiomyocyte senescence remains a major challenge.
Honokiol (Hnk) is an effective ingredient extracted from the bark of Magnolia officinalis and is widely used in Chinese medicine. In previous studies, Hnk was found to have a wide spectrum of pharmacological properties, such as antitumor (10), antibacterial (11) and antihypertensive (12). In addition, recent studies demonstrated that Hnk can protect against pressure overload-mediated cardiac hypertrophy (13), myocardial ischemia/reperfusion injury (14) and Dox-induced cardiomyopathy (15). Several mechanisms underlying the role of Hnk in cardioprotection have been reported, including the inhibition of oxidative stress and apoptosis (16), as well as improvement of autophagy (14) and mitochondrial function (13). In addition, a recent study revealed the association between Hnk and senescence in skin cells (17). However, to the best of our knowledge, it remains unknown whether inhibition of cardiomyocyte senescence is involved in the protective effect of Hnk on the heart. Investigating whether Hnk protects heart cells from senescence may further support the biological effects of Hnk on the heart and uncover its potential clinical applications.
Inflammasomes are multimeric complexes of innate immune receptors and their formation promotes caspase-1 activation, which subsequently results in the processing and secretion of inflammatory cytokines, including interleukin (IL)-1β and IL-18 (18). A number of studies have demonstrated that the activation of the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome directly participates in the regulation of inflammation, oxidative stress and apoptosis in cultured cardiomyocytes (19). Recently, additional studies have demonstrated a pivotal role of the NLRP3 inflammasome in accelerating endothelial cell senescence (20,21). However, the role of the NLRP3 inflammasome in cardiomyocyte senescence remains to be fully elucidated.
Thioredoxin-interacting protein (TXNIP), also termed thioredoxin-binding protein-2/vitaminD3 upregulated protein 1, belongs to the arrestin superfamily and inhibits the disulfide reductase activity of thioredoxin (22). Studies in mammals have demonstrated that TXNIP plays a key role in regulating cell growth (23), apoptosis (24), metabolism (25) and immune responses (26). Previous studies have reported that TXNIP acts as a link between redox regulation and the pathogenesis of senescence (27,28). Additionally, TXNIP may be upregulated during the process of senescence, and upregulation of TXNIP in young cells resulted in typical signs of senescence (29). Furthermore, a study demonstrated that the TXNIP/NLRP3 inflammasome pathway contributes to the senescence of vascular endothelial cells (27), thus providing a novel mechanism for TXNIP/NLRP3 inflammasome-mediated cellular senescence. Despite increasing evidence supporting pivotal roles of TXNIP in the regulation of cardiomyocyte fatty acid oxidation (30) and apoptosis (31), further research is required to determine whether TXNIP and the NLRP3 inflammasome are involved in cardiomyocyte senescence.
The aim of the present study was to investigate the effects of Hnk on Dox-induced cardiomyocyte senescence, and to examine the roles of TXNIP and the NLRP3 inflammasome in Hnk-mediated inhibition of cardiomyocyte senescence.
.../...
Results
Hnk protects H9c2 cardiomyocytes against Dox-induced senescence
According to previous research (37), the present study selected 0.1 µM as the most suitable concentration to induce senescence of H9C2 cardiomyocytes. Dose-response experiments by CCK-8 assay were then performed to identify the optimal concentration of Hnk for the following experiments. It was observed that Hnk suppressed Dox (0.1 µM)-induced reductions in cell viability, with maximum inhibition observed when Hnk was used at 2.5 and 5 µM (Fig. 1A). Therefore, 2.5 and 5 µM were selected to study the effect of Hnk on aging. The 48-h treatment of H9c2 cells with Dox significantly increased the percentage of senescent cells, as assessed by SA-β-gal staining, while this effect was significantly reversed by Hnk treatment at both 2.5 and 5.0 µM (Fig. 1B and C). In addition, p16INK4A and p21 protein expression levels were increased in Dox-treated H9c2 cardiomyocytes compared with the vehicle group, whereas Hnk pretreatment antagonized this effect elicited by Dox (Fig. 1D-F). These results demonstrated for the first time that Hnk can protect H9c2 cardiomyocytes against Dox-induced senescence.
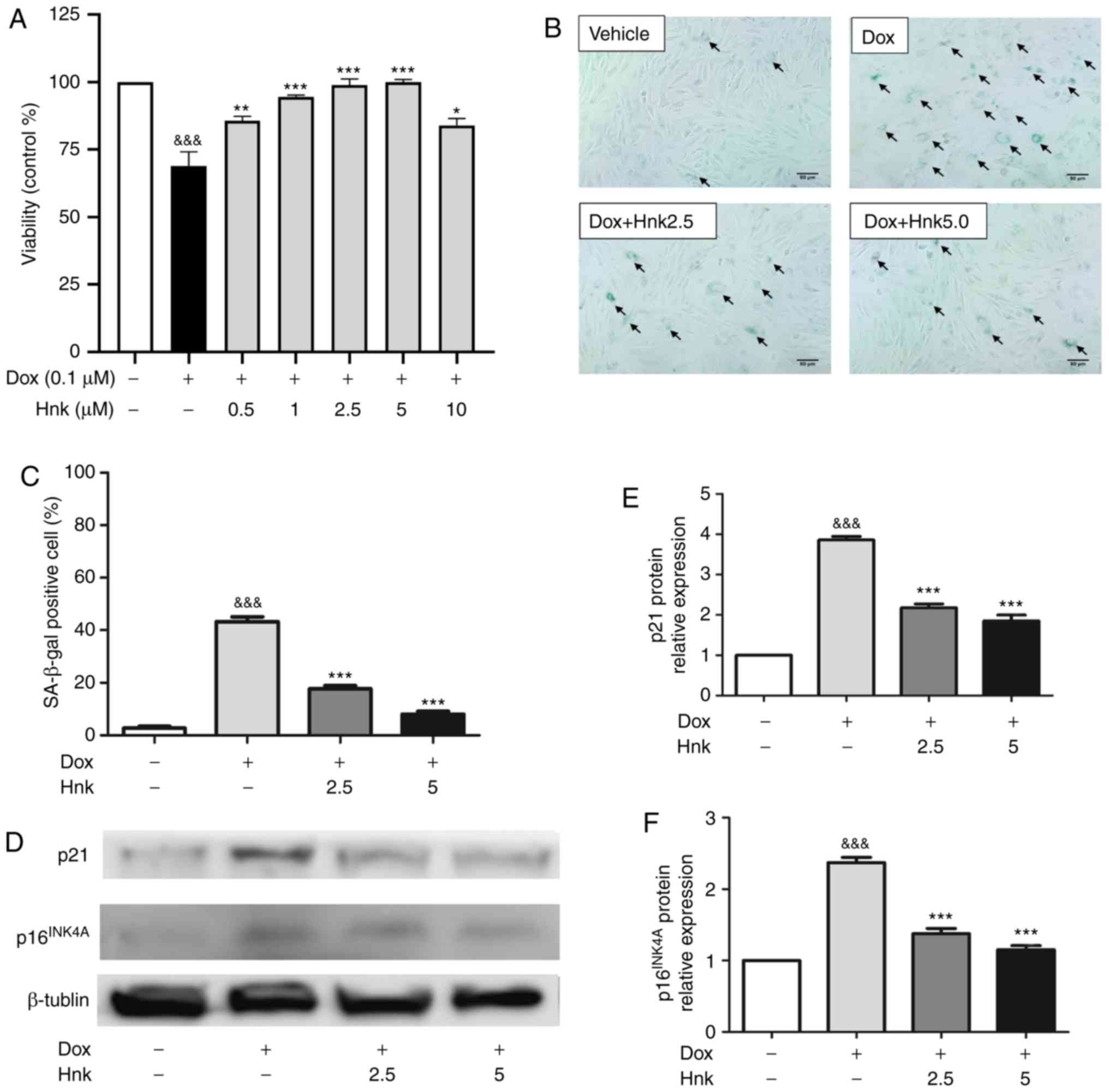
Figure 1 - Honokiol (Hnk) protects H9c2 cardiomyocytes against Dox-induced senescence. (A) H9c2 cardiomyocytes were pretreated with different concentrations of Hnk (0.5-10 µM) for 24 h prior to Dox (0.1 µM) exposure for 48 h, which was followed by a CCK-8 assay to evaluate cell viability. (B) Cultured H9c2 cardiomyocytes were pretreated with Hnk (0, 2.5 or 5 µM), followed by treatment with Dox (0.1 µM) for 48 h. Representative images of the co-staining for SA-β-gal-positive cells (blue; arrows). Magnification ×200. Scale bar, 50 µm. © Percentage of SA-β-gal-positive H9c2 cells. (D) Detection of p16INK4A and p21 protein levels. (E and F) Quantification of p16INK4A and p21 protein levels. The results are representative of at least three independent experiments. &&&P<0.001 vs. vehicle group; *P<0.05, **P<0.01, ***P<0.001 vs. Dox group. SA-β-gal, senescence-associated β-galactosidase; Dox, doxorubicin; Hnk2.5, honokiol 2.5 µM; Hnk5.0, honokiol 5 µM.
Hnk inhibits Dox-induced TXNIP expression and NLRP3 inflammasome activation in H9c2 cardiomyocytes
TXNIP is a recognized activator of the NLRP3 inflammasome (38). In the present study, the protein and mRNA levels of TXNIP were found to be increased significantly in Dox-treated H9c2 cardiomyocytes compared with the vehicle group (Fig. 2A-C). Furthermore, Hnk-pretreatment at both 2.5 and 5.0 µM in cultured H9c2 cells significantly downregulated the expression of TXNIP compared with the Dox group (Fig. 2A-C). As a key modulator of senescence, the NLRP3 inflammasome was analyzed at the mRNA level in H9c2 cardiomyocytes. Dox-stimulated H9c2 cardiomyocytes exhibited higher mRNA levels of NLRP3, caspase-1 and IL-1β compared with the vehicle group (Fig. 2D-F). Consistently, cells pretreated with 2.5 and 5.0 µM exhibited markedly downregulated mRNA levels of NLRP3, caspase-1 and IL-1β compared with the vehicle group (Fig. 2D-F). These results indicated that Hnk was able to inhibit TXNIP expression and NLRP3 inflammasome activation in Dox-treated H9c2 cardiomyocytes.
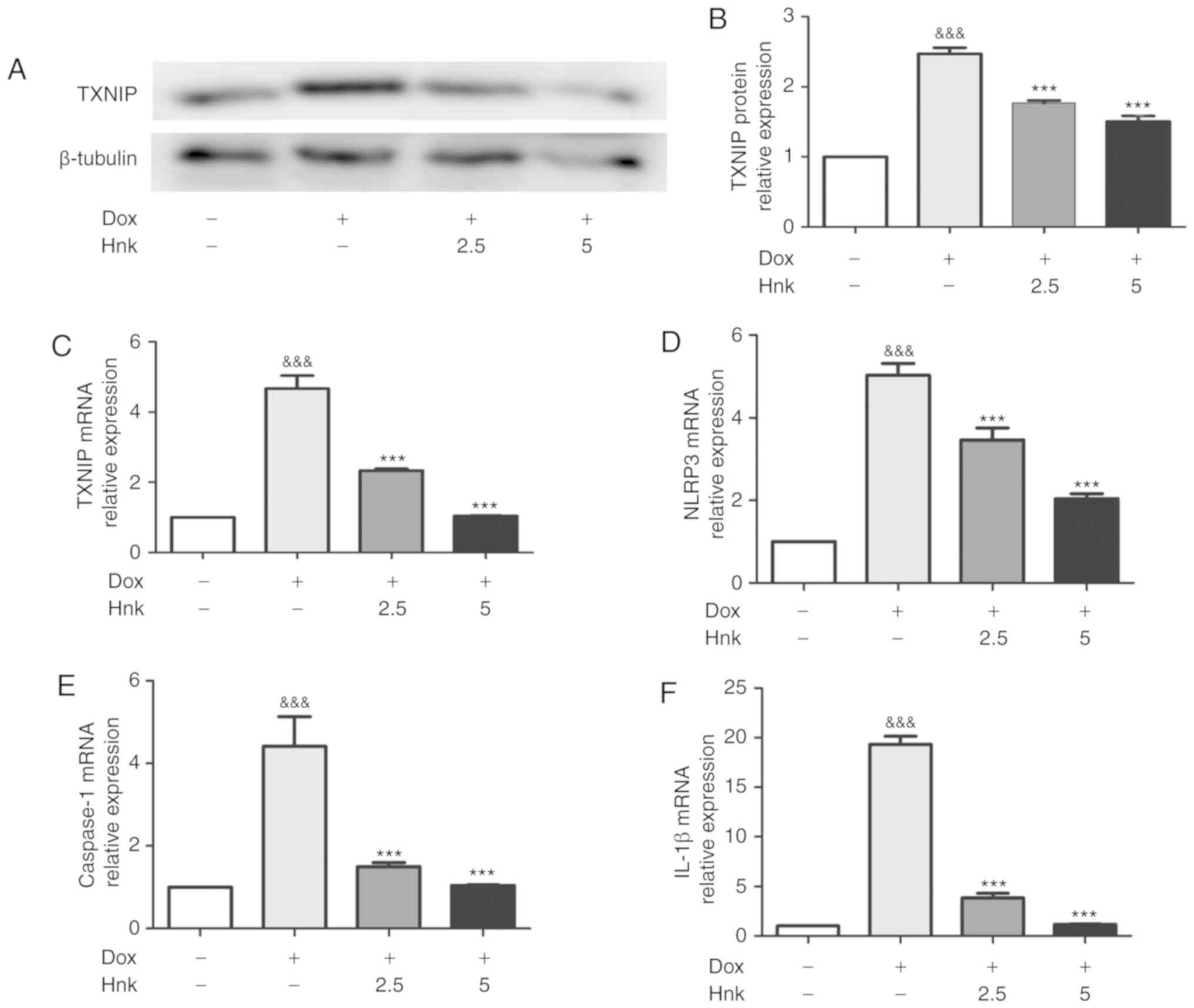
Figure 2 - Honokiol (Hnk) inhibits Dox-induced TXNIP expression and NLRP3 inflammasome activation in H9c2 cardiomyocytes. (A and B) Detection of TXNIP protein levels after no treatment or exposure to Dox with or without prior incubation with Hnk, and quantitative analysis. (C-F) Detection of relative TXNIP, NLRP3, caspase-1 and IL-1β mRNA levels after no treatment or exposure to Dox with or without prior incubation with Hnk. The results are representative of at least three independent experiments. &&&P<0.001 vs. vehicle group; ***P<0.001 vs. Dox group. SA-β-gal, senescence-associated β-galactosidase; Dox, doxorubicin; Hnk2.5, honokiol 2.5 µM; Hnk5.0, honokiol 5 µM; TXNIP, thioredoxin-interacting protein; NLRP3, NOD-like receptor family pyrin domain-containing 3; IL, interleukin.
TXNIP overexpression abrogates the effect of Hnk treatment on Dox-induced senescence of H9c2 cardiomyocytes
To determine whether TXNIP mediates the protective effect of Hnk against Dox-induced H9c2 cardiomyocyte senescence, TXNIP expression was upregulated by adenovirus-mediated gene overexpression (Fig. 4A). Ad-TXNIP partially reversed the inhibitory effect of Hnk on TXNIP expression (Fig. 4B-D). Indeed, the inhibitory effect of Hnk on Dox-induced NLRP3 inflammasome activation was lost when H9c2 cardiomyocytes were pre-infected with Ad-TXNIP (Fig. 4E-G). Furthermore, SA-β-gal staining was performed to detect the cardiomyocyte senescence (Fig. 4H). Pre-infecting H9c2 cells with Ad-TXNIP abrogated the reduction in Dox-induced H9c2 cardiomyocyte senescence attained by Hnk (Fig. 4I). In addition, Hnk almost normalized the expression levels of p16INK4A and p21, and this effect was abolished by pre-infecting H9c2 cells with Ad-TXNIP (Fig. 4J-L). Collectively, these data suggest that Hnk reverses Dox-induced H9c2 cardiomyocyte senescence in a TXNIP/NLRP3 inflammasome dependent-manner.
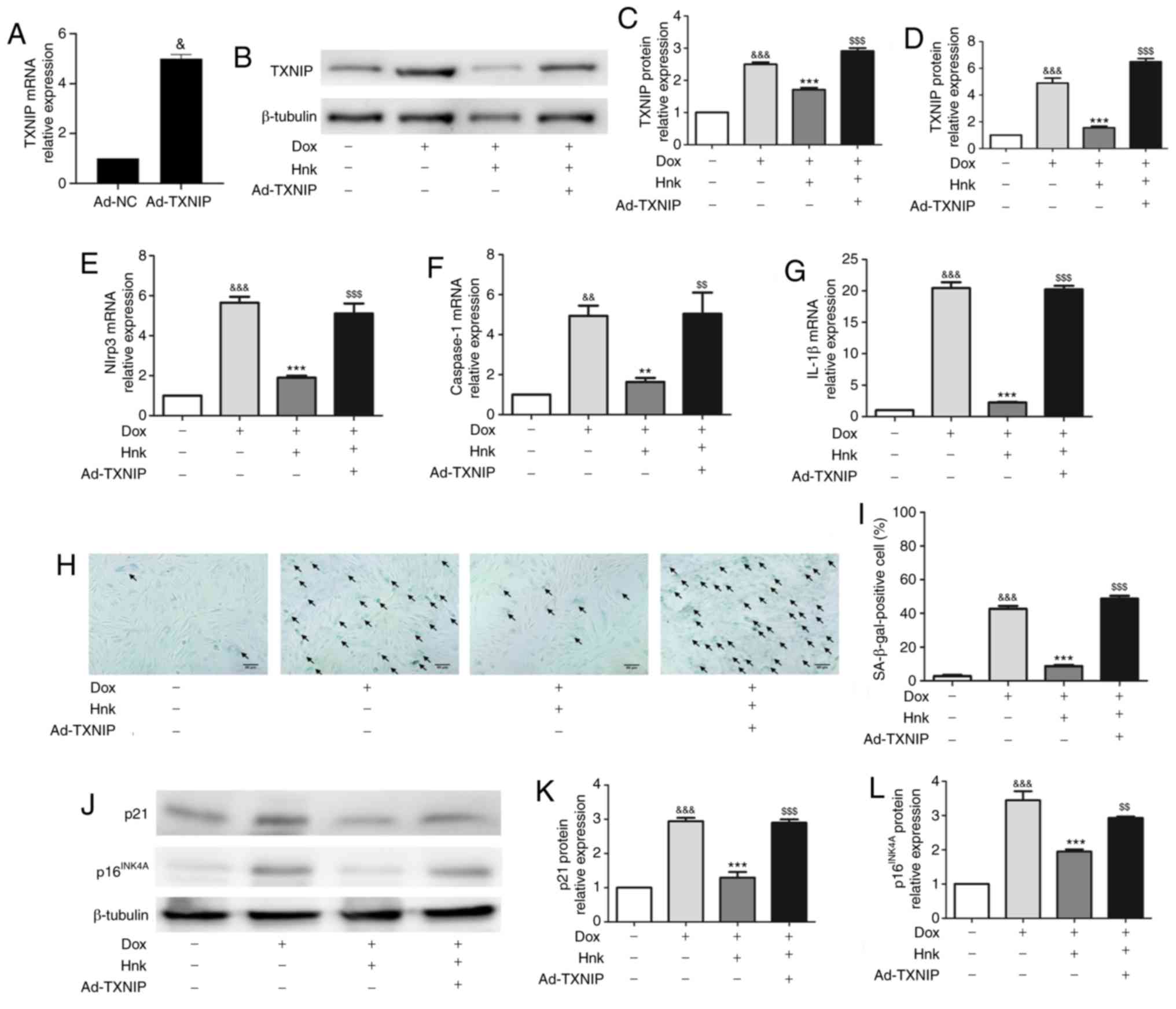
Figure 4 - TXNIP overexpression abrogates the effect of honokiol (Hnk) treatment on Dox-induced senescence in H9c2 cardiomyocytes. Cultured H9c2 cardiomyocytes were transfected with Ad-NC or Ad-TXNIP for 48 h, followed by treatment with Hnk (5 µM) for 24 h and subsequent exposure to Dox (0.1 µM) for 48 h. (A) mRNA levels of TXNIP in H9c2 cells infected with TXNIP overexpression adenovirus. (B and C) Detection of TXNIP protein levels and quantitative analysis. (D-G) Detection of relative TXNIP, NLRP3, caspase-1 and IL-1β mRNA levels. (H) Representative images of co-staining for SA-β-gal-positive cells (blue; arrows). Magnification ×200. Scale bars, 50 µm. (I) Percentage of SA-β-gal-positive H9c2 cells. (J) Detection of p16INK4A and p21 protein levels. (K and L) Quantification of p21 and p16INK4A protein levels. The results are representative of at least three independent experiments. &P<0.05, &&P<0.01, &&&P<0.001 vs. vehicle group; **P<0.01, ***P<0.001 vs. Dox group; $$P<0.01, $$$P<0.001 vs. Dox + Hnk group. Ad, adenovirus; SA-β-gal, senescence-associated β-galactosidase; Dox, doxorubicin; Hnk, honokiol 5 µM; TXNIP, thioredoxin-interacting protein; NLRP3, NOD-like receptor family pyrin domain-containing 3; IL, interleukin.
Discussion
Dox is an anthracycline that is effective against a wide range of tumors (1). Despite its beneficial effects against cancer, the clinical application of Dox is associated with severe cardiotoxicity (2). Dox induces a senescent-like phenotype in cardiomyocytes, with an abnormal pattern of troponin phosphorylation that may result in inefficient cardiac contraction (5). Senescence of cardiac cells has been identified to be crucial for the development of anthracycline-related cardiomyopathy (39). Consistent with these previous findings, the data in the present study suggest that Dox-induced cytotoxicity is mediated by the development of cellular senescence, which is accompanied by increased expression of senescence-related genes and SA-β-gal activity. Therefore, it is of great interest to identify effective therapies that inhibit cardiomyocyte senescence in order to prevent Dox-induced cardiotoxicity.
Hnk is a bioactive natural compound obtained from the genus Magnolia. Hnk has been reported to possess diverse biological properties, including antiarrhythmic, antihy-pertensive and anticancer properties, without appreciable toxicity (12,40,41). It has been demonstrated that Hnk acts as an anti-inflammatory agent by blocking the classical nuclear factor-κB pathway (42). Due to its prominent anti-inflammatory properties, there has been increasing attention on its favorable effects on cardiac performance. Tan et al (14) demonstrated that Hnk post-treatment alleviated myocardial ischemia/reperfusion injury by enhancing autophagic flux and reducing intracellular reactive oxygen species (ROS) production. Furthermore, Pillai et al (13) suggested that Hnk blocked and reversed cardiac hypertrophy in mice by activating mitochondrial sirtuin 3. Although Huang et al (15) demonstrated that Hnk pretreatment could reduce cardiac damage by improving mitochondrial function in Dox-treated mouse hearts, the effects and the underlying mechanisms of Hnk in Dox cardiomyopathy remain to be fully elucidated. The present study confirmed that Hnk was able to attenuate Dox-induced cardiomyocyte senescence, as indicated by the significantly decreased number of SA-β-gal-positive cells, as well as decreased expression levels of p16INK4A and p21. This new evidence supports the previous research demonstrating that Hnk protected Dox-treated mice against cardiotoxicity. Based on these results, Hnk may be of value for suppressing the pathogenesis of Dox-induced cardiomyopathy via modulating cardiac senescence. However, detailed information regarding the precise mechanism underlying the protective effect of Hnk against Dox-induced cardiomyocyte senescence remains to be clearly determined.
.../...
.
Edited by Engadin, 18 December 2019 - 05:25 PM.











































