.
S O U R C E : Life Extension Advocacy Foundation (LEAF)
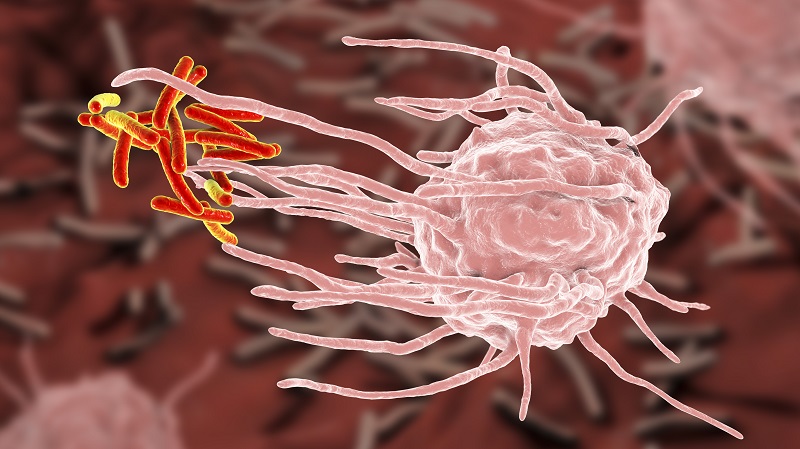
Macrophage engulfing intruder pathogens.
Researchers have found that a protein called TIM-4 is essential to macrophages’ ability to clean up cellular debris and that p38 MAPK, a protein that increases due to aging, inhibits TIM-4.
Inflammaging and immunosenescence
Aging is the primary risk factor for a myriad of chronic age-related diseases, including heart disease, Parkinson’s, Alzheimer’s, and cancer. As well as increasing our risk of developing these diseases, aging also increases our vulnerability to infections and reduces the effectiveness of vaccines.
This is partially because as we grow older, our immune system becomes increasingly dysfunctional and inefficient; this decline of the immune system is a process known as immunosenescence. Detrimental changes occur in both the innate and adaptive immune systems, with the adaptive immune system being more affected than the innate immune system.
Aging is associated with the chronic, low-grade systemic inflammation called inflammaging, a term first coined by Professor Claudio Franceschi in the early 2000s. There are a number of proposed sources of inflammaging, including senescent cells, cell debris, microbial burden, and, of course, immunosenescence.
Inflammaging has a serious impact on many functions in the body; it impairs tissue repair, interferes with cellular communication, stem cell function, and can cause the immune system to become deregulated and behave inappropriately.
The immune system relies on acute inflammation during the immune response to fight invading pathogens and to facilitate wound healing. This triggers cell turnover and tissue repair and is, in general, a desirable reason for inflammation. However, in direct contrast to this, the chronic, low-grade, persistent background of inflammation that inflammaging produces leads to poor tissue repair and degeneration.
Inflammaging also contributes to the development of age-related diseases and is instrumental in driving aging in general. The organs and tissues of older people have high levels of inflammatory cytokines, such as IL-6, IL-1β, TGF-b, and TNF-α, which are known to interfere with anabolic signaling, including insulin and erythropoietin signaling, thus contributing to the development of sarcopenia and diabetes.
The impact of inflammation on the behaviour of the innate immune system, particularly immune onset and resolution, is less clear, and researchers have published a new study that sheds more light on it [1].
The researchers showed that while the initial inflammatory immune response is similar in young and older people, the immune resolution phase is significantly impaired in the elderly. This was due to the reduction of T cell immunoglobulin mucin receptor-4, also known as T-cell membrane protein 4 (TIM-4), a protein in humans that is encoded by the TIMD4 gene. TIM-4 expression by macrophages plays an important role in their homeostatic maintenance and allows them to engulf cell debris and other waste. Essentially, the less TIM-4 there is, the worse macrophages become at removing the garbage that our bodies accrue during operation.
The researchers show that TIM-4 levels fall in the elderly due to an increased level of macrophage p38 mitogen-activated protein kinase (MAPK or MAP kinase). Therefore, the research team gave elderly people an orally active p38 inhibitor to reduce the activity of p38. This restored TIM-4 expression and sent the macrophages back to work eating up the previously ignored cell debris. They found that following this inhibition of p38, the immune resolution response between old and young people was similar, effectively rejuvenating this aspect of immune function.
Increasing age alters innate immune–mediated responses; however, the mechanisms underpinning these changes in humans are not fully understood. Using a human dermal model of acute inflammation, we found that, although inflammatory onset is similar between young and elderly individuals, the resolution phase was substantially impaired in elderly individuals. This arose from a reduction in T cell immunoglobulin mucin receptor-4 (TIM-4), a phosphatidylserine receptor expressed on macrophages that enables the engulfment of apoptotic bodies, so-called efferocytosis. Reduced TIM-4 in elderly individuals was caused by an elevation in macrophage p38 mitogen-activated protein kinase (MAPK) activity. Administering an orally active p38 inhibitor to elderly individuals rescued TIM-4 expression, cleared apoptotic bodies and restored a macrophage resolution phenotype. Thus, inhibiting p38 in elderly individuals rejuvenated their resolution response to be more similar to that of younger people. This is the first resolution defect identified in humans that has been successfully reversed, thereby highlighting the tractability of targeting pro-resolution biology to treat diseases driven by chronic inflammation.
.../...
T O A C C E S S T H E R E S T O F T H E A R T I C L E , P L E A S E V I S I T T H E S O U R C E .
.
Edited by Engadin, 15 April 2020 - 07:51 PM.









































