.
S E C O N D A R Y S O U R C E : Life Extension Advocacy Foundation (LEAF)
A new study may shed some light on how aging and circadian rhythms may be linked by discovering a role for nicotinamide adenine dinucleotide (NAD+) in preventing the age-related disruption of circadian rhythms in mice.
A circadian rhythm is an automatic process that regulates a cycle of behavioral changes and repeats roughly every 24 hours. The most well-known example of a circadian rhythm is sleeping at night and being awake during the day, but it can refer to any biological process that has an internal, entrainable rhythm based around a 24-hour period.
It is well documented that as we age, our circadian rhythms can become disrupted, which may contribute towards the development of age-related diseases. While researchers have been aware of circadian rhythms for a long time now, the actual molecular workings and mechanisms linking them with aging are still somewhat of a mystery.
Thankfully, new research may shed some light on how the two could be linked, and this research may also have relevance to humans.
What is NAD+?
Nicotinamide adenine dinucleotide is a coenzyme found in all living cells. It is a dinucleotide, which means that it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base, and the other contains nicotinamide.
In metabolism, NAD facilitates redox reactions, carrying electrons from one reaction to another. Therefore, NAD is found in two forms in the cell; NAD+ is an oxidizing agent that takes electrons from other molecules in order to become its reduced form, NADH. NADH can then become a reducing agent that donates the electrons it carries. The transfer of electrons is one of the main functions of NAD, though it also performs other cellular processes, including acting as a substrate for enzymes that add or remove chemical groups from proteins in post-translational modifications.
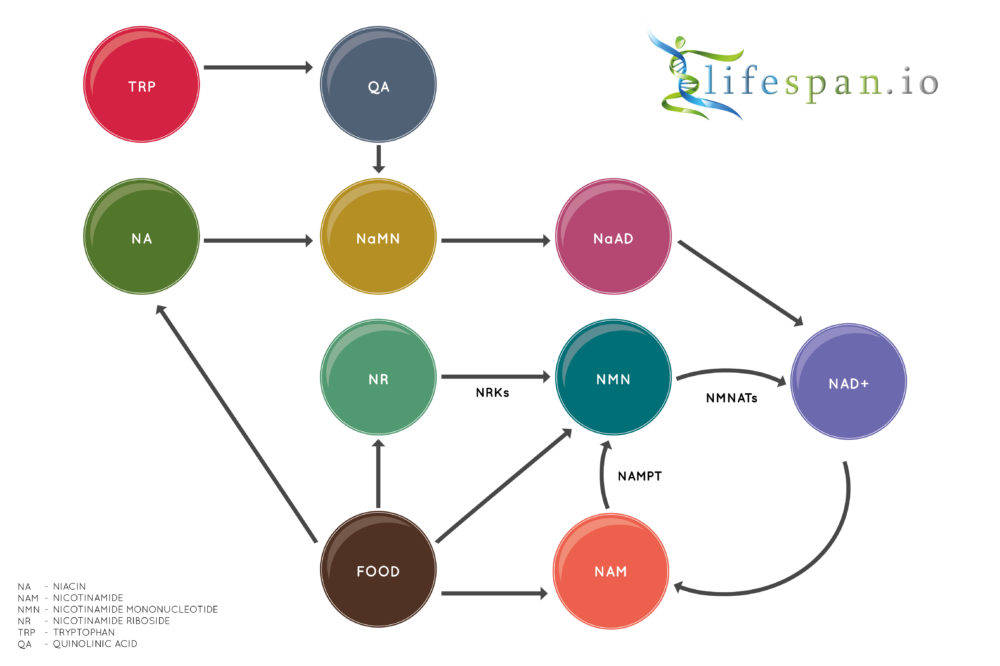
NAD+ biology has seen a great deal of interest in the last few years, partially due to the discovery of two precursors of NAD+ biosynthesis, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), which both increase NAD+ in multiple tissues. NAD+ is also a cofactor for sirtuin deacetylases, which are known to promote healthspan and lifespan.
As we age, cellular levels of NAD+ decline, largely (if not wholly) due to the chronic systemic age-related inflammation known as inflammaging. Previous animal studies have shown that the use of NAD+ precursors can promote more youthful behavior in aged mice as well as reduce some aspects of aging.
The NAD+ and circadian rhythm connection
Researchers have published the results of a new study suggesting a role for NAD+ in resisting age-related circadian rhythm disturbance using the NAD+ precursor nicotinamide riboside [1].
The mice were given NR in their drinking water for a period of 4 months; they then examined circadian-regulated gene expression in the mouse livers. The results showed that the gene expression patterns of around half of the circadian-regulated liver genes were altered in a beneficial manner when NAD+ levels were increased using NR.
The connection that NAD+ and circadian rhythms have are through sirtuins, of which sirtuin 1 (SIRT1) regulates circadian rhythms. When there is sufficient NAD+ available, the body is able to regulate circadian rhythm properly, but, inevitably, as aging steadily creeps in and the level of NAD+ production declines, SIRT1 can no longer work with it to regulate circadian rhythm. The paper is worth a read as it explains the exact mechanism by which this works, but this is a simple summary of what happens:
Disrupted sleep-wake and molecular circadian rhythms are a feature of aging associated with metabolic disease and reduced levels of NAD+, yet whether changes in nucleotide metabolism control circadian behavioral and genomic rhythms remains unknown. Here, we reveal that supplementation with the NAD+ precursor nicotinamide riboside (NR) markedly reprograms metabolic and stress-response pathways that decline with aging through inhibition of the clock repressor PER2. NR enhances BMAL1 chromatin binding genome-wide through PER2K680 deacetylation, which in turn primes PER2 phosphorylation within a domain that controls nuclear transport and stability and that is mutated in human advanced sleep phase syndrome. In old mice, dampened BMAL1 chromatin binding, transcriptional oscillations, mitochondrial respiration rhythms, and late evening activity are restored by NAD+ repletion to youthful levels with NR. These results reveal effects of NAD+ on metabolism and the circadian system with aging through the spatiotemporal control of the molecular clock.
Edited by Engadin, 25 May 2020 - 10:05 PM.














































