.
O P E N A C C E S S S O U R C E : Antioxidants
Abstract
Terpenes and terpenoids are the largest groups of plant secondary metabolites. However, unlike polyphenols, they are rarely associated with geroprotective properties. Here we evaluated the conformity of the biological effects of terpenoids with the criteria of geroprotectors, including primary criteria (lifespan-extending effects in model organisms, improvement of aging biomarkers, low toxicity, minimal adverse effects, improvement of the quality of life) and secondary criteria (evolutionarily conserved mechanisms of action, reproducibility of the effects on different models, prevention of age-associated diseases, increasing of stress-resistance). The number of substances that demonstrate the greatest compliance with both primary and secondary criteria of geroprotectors were found among different classes of terpenoids. Thus, terpenoids are an underestimated source of potential geroprotectors that can effectively influence the mechanisms of aging and age-related diseases.
1. Introduction
Terpenoids are the largest group of plant secondary metabolites [1]. There are tens of thousands of naturally occurring hydrocarbons and they are one of the most structurally diverse classes of natural compounds. Terpenoids consist of C = 5, 10, 15, 20…, n > 40 carbon units and are classified as hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes or carotenoids (C40), and polyterpenes (Cn, n > 40) [1]. Extensive biological investigations revealed a wide range of pharmacological and physiological activities of terpenoids and their derivatives [2,3,4]. However, terpenoids are rarely associated with anti-aging properties and may be underestimated as potential geroprotectors.
Geroprotectors are the pharmacological agents that decrease the rate of aging and extend lifespan. Despite the fact that terpenoids are the broad class of compounds, only a few of its representatives are well-known geroprotectors [5]. However, they are attracting increasing interest and such a systematic review of geroprotectors of various classes of terpenoids is necessary.
We proposed a set of primary and secondary selection criteria for potential geroprotector [6]. Primary criteria that should be met:
1. The life extension in experiments with wild type animal models. The geroprotector should prolong the life of the model beyond the intact maximum lifespan, protecting it from one or more mechanisms of aging.
2. Improvement of molecular, cellular, and physiological biomarkers to a younger state or slow down the progression of age-related changes in humans.
3. Most potential geroprotectors are preventive only when applied at relatively high concentrations. The lifespan-extending dose should be several orders of magnitude less than the toxic dose.
4. Minimal side effects at the therapeutic dosage at chronic application.
5. The potential benefit of taking a geroprotector may come after a long period. Potential geroprotectors should initially improve some parameters of health-related quality of life: physical, mental, emotional, or social functioning of the person.
Secondary selection criteria for potential geroprotector:
6. The target or mechanism of action of the geroprotector that extends the lifespan of the model should be evolutionarily conserved.
7. Reproducibility of geroprotective effects on different model organisms increases the possibility that effects will also be discovered in humans, even in the absence of a known conserved target.
8. Candidate geroprotectors should be able to delay the progress of one or several age-associated diseases in humans.
9. Potential geroprotectors should increase organism resistance to unfavorable environmental factors.
This review discusses terpenoid compounds belonging to different classes of this large group of substances, in terms of meeting the criteria for potential geroprotector and their potential for clinical use in relation to age-dependent diseases.
2. Extraction and Analysis of Terpenoids
Detection and structural identification play an important role in elucidating potential activities and developing therapeutic approaches to natural geroprotectors. Currently, advancement has been made in the determination of structure and studying of the chemical features of terpenes and terpenoids, as well as methods for their extraction. These methods are constantly improving, and new approaches are being developed [7].
All terpenes and terpenoids contain a hydrocarbon skeleton, which is formed from five-carbon precursors (isopentenyl diphosphate and dimethylallyl diphosphate) and polymerizes with the formation of prenyl diphosphates of various lengths. As a result of the removal of the diphosphate group, the intermediate allyl cations can be subjected to a series of chemical cascades with the formation of various compounds with linear and/or cyclized hydrocarbon chains, which are then further modified by the addition of various functional groups and adducts [2,3,4]. This feature of terpenes and terpenoids creates their remarkable chemical diversity and requires an appropriate approach for extraction, detection, and purification [8,9,10].
The preparation and analysis of natural compounds include four stages: (1) the release of biologically active compounds; (2) extraction; (3) purification of a target substance from an extract; (4) identification of the chemical structure of a target compound. The use of specific methods is determined depending on the size and complexity of a molecule, its physical properties (polarity, volatility), chemical properties, and some other parameters [7]. The polarity of a molecule is the most important feature that should be considered when determining the method of extraction, analysis, and purification of a substance [8,9,11].
Most primary terpenes without chemical modifications are non-polar. Some of them with the smallest molar mass (especially mono- and sesquiterpenes) can be volatile [12]. Non-volatile non-polar terpenes are extracted with hexane or other non-polar solvents [9]. In addition, to extract this fraction, silica can be used as a stationary phase. For the analysis of the obtained samples of non-polar terpenes, purification of the target molecules, and their structural identification, gas chromatography is used [9]. Additionally, thin layer chromatography (for more accurate identification of specific fractions with the target molecule) and high-performance liquid chromatography (for thorough purification) are applicable [7].
Isolation and purification of volatile non-polar terpenes have limitations associated with the need for their capture and the difficulties of separating substances from each other [7,8]. There is a molecular capture technique and novel approaches, such as solid-phase microextraction [13,14] or microwave-assisted extraction [15]. For structural identification, gas chromatography is also used [12].
The process of extraction and analysis of compounds with modifications requires other methods because they are polar molecules with greater variability of chemical properties. The degree of polarity depends on the type of modifications and their quantity. Modifications by the addition of methyl or hydroxyl groups provide a relatively low polarity of the compounds. For extraction, a suitable approach is the use of hexane (or polar methanol) as a solvent, but for analysis, liquid chromatography. However, it is possible to use gas chromatography, supplemented by derivatization, as well as thin-layer chromatography [7].
Modifications such as acylation, aroylation, glycosylation, and the addition of other functional groups increase the size and polarity of triterpenoids. For the extraction of such fractions, it is recommended to use polar solvents, in particular, methanol (or alternative methods, such as extraction with ionic liquid), and for analysis, high-performance liquid chromatography/electrospray tandem mass spectrometry [16,17]. Additional methods may be required to prepare the samples, depending on the chemical structure of specific terpenes and terpenoids [7,11].
3. Terpenoids as Potential Geroprotectors
3.1. Monoterpenes
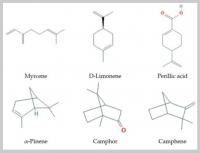
Monoterpenes (Figure 1) are isoprene dimers; they have the composition C10H16. These are easily volatile compounds with a pleasant smell that form the basis of essential oils of plants. According to the structure, monoterpenes are divided into two large groups: acyclic, with an open carbon chain (for example, myrcene, ocimene) and cyclic, which can contain both one cycle (limonene) and several (fennenes, pinenes); some bicyclic monoterpenes contain cyclopropane cycles (sabinene).
Figure 1
Some monoterpenes that increase lifespan and delay age-related diseases.
3.1.1. Natural Sources
These substances are the main components of essential oils of fruits and the volatile turpentine fraction of conifer oleoresins. Camphene is found in the juniper oil, pettigrein—in the pine oil; dipentene—in oils of bergamot, coriander, sweet dill, lemon; limonene—in oils of bergamot, cumin, carrot, sweet dill, lemon, neroli, orange; pinene—in oils of coriander, cypress, eucalyptus, sweet dill, pine, rosemary; sylvestren—in oils of cypress, pine, and many other tree oils. Monoterpenes myrcene and ocimene are contained in essential oils of hops and laurel. Monoterpene alcohols, such as geraniol, are the main components of essential oils of rose, geranium, and other flower essences. Aldehydes (geranial) have a citrus smell and are contained in lemon essential oils. Camphor, a bicyclic monoterpene, is one of the major constituents of essential oils from rosemary, lavender, and sage [18]. In different parts of pine, the following were found: γ-terpinene and β-pinene—in the needles; limonene—in the bark; α-pinene and limonene—in the pollen [19]. Monoterpenes limonene is contained in the lemon oil and turpentine, and is a part of the oil of cumin. The highest content of limonene was found in representatives of the genus Citrus (Rutaceae) [20,21].
3.1.2. Lifespan Extension on Different Models
The hormetic-like effect of limonene was found in the Mediterranean fruit fly (Ceratitis capitata) model: despite the frank toxicity of limonene in high doses (LD90—39.74 nL per male and 75.51 nL per female), low doses (LD20—3.47 nL per male and 12.26 nL per female) increased the lifespan and female fertility in the case of protein-free diet [22]. Small concentrations of limonene (0.011 and 0.046 mM) also significantly increase both average and maximum lifespan of Drosophila melanogaster [23]. Exposure of the olive fruit flies (Bactrocera oleae) by the aroma of α-pinene, which is present in both olive fruit and leaves, increased the lifespan in males and fecundity in females under dietary restricted conditions [24]. However, studies conducted on the Mediterranean fruit fly did not confirm the pro-longevity effect [22].
3.1.3. Effects on Stress-Resistance
Low doses of camphor could be beneficial, inducing neurohormesis [25] or anti-tumor activity [26]. Camphor as a component of cosmetics can delay skin aging, decreasing the activity of elastase, elevating collagen expressions, activating proliferation of human primary dermal fibroblasts, and attenuating cellular senescence [18]. It has anti-mutagenic effects in small doses [27]. α-Pinene attenuated UV-induced photoaging by inhibiting the expression of matrix metalloproteinases in mouse skin [28]. Antioxidant properties were found for menthol [29] and γ-terpinene [30], these compounds prevent peroxidation of lipids and fatty acids. In Caenorhabditis elegans, the antioxidant activity of the mint essential oil was comparable to ascorbic acid [29]. Perillic acid showed protective properties in radiation-induced oxidative stress [31]. A mixture of geraniol and camphene prevented mitochondrial dysfunction, oxidative stress, and the release of apoptotic proteins in the liver during the nimesulide poisoning in rats [32]. The methanol extract of fennel seeds containing L-limonene softened oxidative stress and protected mouse cells from the damage caused by active forms of oxygen [33].
3.1.4. Toxicity and Side Effects
High doses of camphor have pronounced toxicity. In the Ames test, monoterpenoids (camphor, 1,8-cineol, citral, citronellol, menthol, except for terpineol) showed no mutagenic properties [34]. Low doses of monoterpenes, such as camphor, eucalyptol, and thujone, have a cytoprotective and antimutagenic effect, however, in large doses, their effects are opposite [27]. The products of the interaction of limonene with oxygen (oxygen hydroperoxides) could be aromatic allergens [35].
3.1.5. Life Quality Effects
Monoterpenes are often partially responsible for the aroma or odor of plants and are major odoriferous compounds of many flowers and fruits. Esters of terpene alcohols (citronellol, geraniol, nerol, farnesol, linalool, perillyl alcohol, menthol, borneol, carveol) are described as highly potent, reversible, and low toxic skin penetration enhancers [36]. D-limonene reduces overall stress levels and improves markers of inflammation [37,38,39]. D-limonene in Wistar rats caused the intense and persistent bradycardia associated with hypotension. In the in vivo model of arrhythmia, D-limonene (10 mg/kg) reduced the heart rate and arrhythmia [40]. In experiments with Oreochromis niloticus, Citrus bergamia peel oil containing limonene and linalool was added to the fish diet. Highest levels of total protein and lowest levels of serum cholesterol and triglycerides were observed in fish treated with 0.5 g per 100 g of bergamot oil, and fish growth rates were significantly increased [41].
3.1.6. Suppression of Pro-Aging or Activation of Anti-Aging Molecular Targets or Pathways
Hormesis pathways activated by phytochemicals include NRF2 and FOXO transcription factors that stimulate the production of antioxidant enzymes, protein chaperones, and neurotrophic factors [25]. Camphor induced the proliferation of primary human skin fibroblasts via PI3K/AKT and ERK signaling pathways. It attenuated an increase of the β-galactosidase (SA-β-gal) activity associated with aging, induced the expression of collagen (IA, IIIA, IVA types) and elastin in primary human dermal fibroblasts [18]. Myrcene ameliorates human skin extrinsic aging via decreasing the production of ROS, MMP-1, MMP-3, and IL-6, and increasing of TGF-1 and type I procollagen secretions. Myrcene treatment reduces the induction of mitogen-activated protein kinase (MAPK)-related signaling molecules such as p-ERK, p-p38, and p-JNK, and AP-1 [42]. Abisil, a substance of terpenes of Abies sibirica enchances the activity of a cellular energy sensor—AMPK—in mice [43].
3.1.7. Effects on Age-Related Diseases
Citronellol decreased hyperglycemia in streptozotocin-induced diabetic rats. The addition of citronellol to the STZ diet of rats positively influenced the maintenance of normal histological manifestation of liver cells and insulin-positive β-cells [44]. In a study of effects of limonene and perillic acid in C57BL/6 mice, a significant (65% and 67%) inhibition of the metastatic tumor formation was revealed [45]. Immunomodulatory activity (increase in total leukocyte count) was detected in Balb/c mice after the consumption of limonene and perillic acid [46]. D-limonene has chemopreventive activity against mammary, skin, liver, lung, and forestomach cancer in rodents [47]. D-limonene and its derivatives have chemotherapeutic and chemoprophylactic efficacy in cancer in various preclinical model systems [48]. On the cellular model of osteoarthritis, it was shown that myrcene has significant anti-inflammatory and anti-catabolic effects on human chondrocytes and is able to slow down the destruction of cartilage and the development of osteoarthritis. Myrcene and limonene prevent the increased expression of non-cartilage specific collagen I induced by IL-1β [49]. α-Terpineol has antitumor activity and acts by suppressing the transmission of NF-κB signals [50]. The protective effect of α-terpineol against disruption of synaptic plasticity of the hippocampus and spatial memory after transient cerebral ischemia in rats was revealed by facilitating long-term potentiation and suppressing lipid peroxidation in the hippocampus [51]. γ-Terpineol inhibited cell growth and caused apoptosis in human Bel-7402 cancer cells. A possible anti-cancer mechanism of γ-terpineol on human hepatoma cells is the induction of cellular apoptosis suppressing the growth of tumor cells [52]. Monoterpenes inhibit cell growth, cell cycle progression, and expression of the cyclin D1 gene in human breast cancer cell lines, and cause dose-dependent inhibition of cell proliferation [53]. Camphene reduces plasma cholesterol and triglycerides in rats with hyperlipidemia [54]. Terpenes of Abies sibirica affect molecular pathways associated with cancer and aging in human cells [5], induce apoptosis and inhibit proliferation in tumor cells in vitro, suppress tumor growth and angiogenesis in vivo [43].
3.1.8. Additional Activities
The antibacterial activity of the essential oil of Citrus hystrix with a concentration of 2% (by weight) showed a strong inhibitory effect against Bacillus subtilis and Escherichia coli [21]. Trichophyton rubrum is a fungus that causes chronic dermatophytosis in humans. Geraniol and citronellol exhibit antimicrobial properties damaging cell wall and cell membrane of T. rubrum by inhibiting ergosterol biosynthesis [55]. The essential oil of Santolina impressa, which includes β-pinene, 1,8-cineole, limonene, camphor, has a fungicidal effect on Cryptococcus neoformans, Epidermophyton floccosum, and Trichophytum rubrum [56]. The essential oil of leaves of Psidium guajava inhibited pathogenic human bacteria Curvularia lunata [57]. Essential oils from various aerial parts of Pinus eldarica show antibacterial properties against E. coli (essential oil from pollen). Essential oil from the cortex inhibited the growth of Candida albicans and Staphylococcus aureus, as well as a decreased growth of S. aureus, under the influence of the essential oil from the needles [19].
3.2. Sesquiterpenes
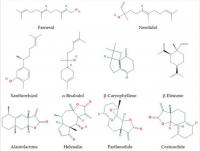
Sesquiterpenes (Figure 2) are C15-terpenoids built from three isoprene units. They are found particularly in higher plants and in many other living systems such as marine organisms and fungi. Usually sesquiterpenes are hydrocarbons or have oxygenated forms including lactones, alcohols, acids, aldehydes, and ketones.
Figure 2
Some sesquiterpenes that increase lifespan and delay age-related diseases.
.../...
.