With so many health and disease outcomes connected to the biological aging processes, research into quantifying and tracking aging has surged in recent years due to the work of Steve Horvath, Morgan Levine, and others. Currently, epigenetic analysis of biological aging is thought to be the most comprehensive and accurate measurement of aging that is currently available.
Through interpreting epigenetic methylation data, most aging algorithms output the lifetime state of an individual’s biological age as an age estimate. With this age, you can determine if you are undergoing accelerated or decelerated aging. Obviously, as age is the number one risk factor for most chronic diseases, accelerated aging would predispose you to these conditions.
Conversely, decelerated aging would be protective of these age-related outcomes. Knowing where you stand can be helpful, as it allows you to make changes that affect your risk factors. However, just knowing your overall age still leaves something to be desired, as it can lead to limitations in our understanding of aging.
Finding a better snapshot
One major limitation is that we are unable to ascertain our current trajectory of aging. For instance, someone’s prior history of poor nutrition or diet might not give them an appropriate idea of how they are aging right at the moment. How much of our aging is from things that have happened in the past, compared to recent changes? Because most of the Horvath aging clocks have approximately 40% heritability, how much is due to our genetics or ancestry?
While the overall picture of age might be useful for knowing risk factors and predicting the outcome of our bodies’ aging phenotypes, it is limited in how it can help us with treatments in interventions due to its scope and precision.
Is there an algorithm that can give us a better snapshot in time?
The Dunedin Pace of Aging measurement is poised to revolutionize aging measurements by offering an instantaneous look at a person’s current pace of aging.

The DunedinPACE algorithm acts like a speedometer, showing how accelerated or slowed a person’s aging is at the time they took the test, with only one test. This provides a much more precise method to track and understand the impact of aging interventions along with how changes in disease states or lifestyle impact epigenetic aging.
The creation of DunedinPACE: 50 years in the making
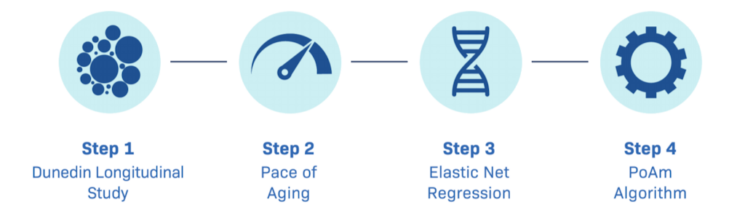
The DunedinPACE algorithm was created by researchers from Duke, Columbia, and the University of Otago. Duke professors Terrie Moffitt and Acshalom Capsi headed a team of six, who finished developing this tool in early 2021.
However, this research had been building for over 42 years.
It started in 1975 with the creation of the Dunedin cohort. Through the Dunedin Multidisciplinary Health and Development study that started in 1972, researchers studied 1037 New Zealanders from birth. The idea of this cohort was to track people over the course of their lifetime in a biobank setting and to measure multiple different lab values and phenotypic measures, such as DEXA imaging, dental evaluation, MRI, and many aging phenotypes. As a result, this cohort is perfect to study the epigenetic effects of aging and one of the only cohorts where these phenotypic markers exist.
When Dr. Moffit’s lab first applied for funding for research, it was interested in the aging rates found in young individuals. However, the National Institute of Aging denied its first attempts at funding. The NIA thought that there would probably not be any variation in aging, and if there was, the agency thought that it probably wouldn’t be significant. Despite this, the investigation continued.
After several periods of investigation, this research culminated in the creation of the DunedinPACE algorithm, which is able to measure this rate of aging. It was created by using phenotypic data to create a pace of aging, and with that, the team was able to regress the data against methylation factors.
Comparisons to first- and second-generation clocks
The generation of epigenetic age algorithms is usually defined by what they are regressed against. Regression is a mathematical method of drawing a quantitative connection between two variables. For instance, the first-generation algorithms are regressed against chronological age in order to predict age. However, if you want to just accurately predict age in clinical medicine, you can usually just ask for a birthdate.
That is how the second-generation algorithms started. The second-generation algorithms are regressed against phenotypic markers, such as PhenoAge, which was regressed against a phenotypic score influenced by blood levels and morbidity.
This allows us to more accurately predict outcomes associated with age. With GrimAge, the algorithm was regressed against proteomic values and, ultimately, time until death so that we can predict lifespan.
A new third-generation algorithm
The DunedinPACE algorithm has been theorized to be the first third-generation algorithm, as it reveals a new metric of aging. Additionally, due to the 43-year longitudinal dataset used to create it, it has many features that might have been considered limitations of previous aging clocks.
For instance, unlike many of the other clocks, it is not confounded by generational exposures. The previous clocks were created using single point-in-time measurements from many publicly accessible biobanks. Some of these samples were taken over various years and different generations when exposures were, most likely, not the same.
For instance, we are now no longer exposed to leaded gasoline. However, we probably are more exposed to plastic chemicals and antibiotics. In the Dunedin cohort, all of the individuals are of the same generation, so these exposures are mostly controlled for, and the aging signals are more easily detectable.
Additionally, one general limitation of aging clocks is intra-sample variability. For example, if you were to test the same blood draw twice to assess age, would we get the same answer? Or will one say 50 and the other 55? Unfortunately, the epigenetic clocks published to date have been relatively imprecise. However, the DunedinPACE algorithm shows a higher reliability.
Maximizing reliability
One way to measure reliability is via a measurement called Intra Class Correlation (ICC). ICC is a descriptive statistic that can be used when quantitative measurements are made on units that are organized into groups. It describes how strongly units in the same group resemble each other.
Measurements closer to one are generally more highly correlated. When comparing DNA samples tested twice from 183 patients, DunedinPACE yields an ICC of .90 vs. Horvath’s .65, Hannum’s .62, PhenoAge’s .77, and GrimAge’s .78. This shows that DunedinPACE has less variation between samples, and, as a result, it might be better for comparing treatment interventions in small periods of time.
With better sensitivity, detection, shorter retest cycles, and fewer subjects required to research significant insights, DunedinPACE is quickly becoming a key monitoring tool in personalized aging treatment programs.
With real-time feedback on an individualized basis, healthcare practitioners can even test the impact of certain supplements or diets on a single person. Since two patients may have drastically different responses to the same medication or supplement, healthcare practitioners can instead gauge how a single patient’s aging is being influenced by a certain supplement to make aging a far more personalized experience.
What outcomes can DunedinPACE predict?
Because the Dunedin Pace of Aging algorithm was trained on detailed data from a wide array of ages and health outcomes, it became one of the most highly predictive algorithms for health and disease. Even from very early in life, DunedinPACE was able to correctly identify accelerated aging and predict individuals who had a greater long-term risk of poor health, chronic disease, and dying earlier. Similarly, individuals who the DunedinPACE algorithm identified as aging more slowly, later performed significantly better on tests of balance, strength, and mental ability.
Measured with the algorithm, individuals whose pace of aging value was even slightly accelerated beyond the ‘normal’ bounds (a rate of aging above 1 biological year per chronological year) had increased their risk of death in the next 7 years by 56% and increased their risk of chronic disease diagnosis by 54%.
Phenotypic changes associated with and predicted by accelerated aging measured with DunedinPACE include:
- Facial phenotypes
- Balance & coordination
- Grip strength
- Cortical thickness & brain surface area
- Eyesight
- Hearing
- Lung function
- Dental health
- Bone density
- Additional values
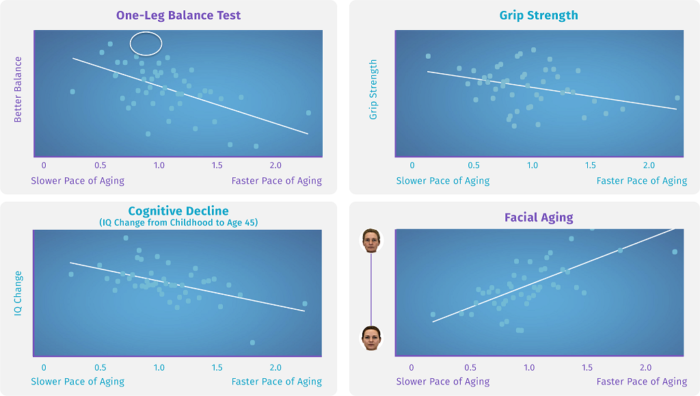
Each data point represents 20/2938 cohort members at age 45. You can see on the top left that fast agers have poor balance as adults. On the top right, the faster agers also had weaker grip strength already by age 45. On the bottom left, fast agers had more decline on tested cognitive function from childhood to age 45. On the bottom right, fast agers even had faces which researchers rated as older looking at age 45.
Individuals in this render all share the same birth year – 1972. Overlaying the faces from the 10 slowest aging, 10 average-aging, and 10 fastest-aging individuals as detected by the DunedinPACE algorithm, it’s clear that outward facial aging is connected to the methylation biomarkers that DunedinPACE is tracking.

What changes or influences DunedinPACE?
Children who grow up socioeconomically disadvantaged face an increased burden of disease and disability throughout their lives. One hypothesized mechanism for this increased burden is that early-life disadvantage accelerates the biological processes of aging, increasing vulnerability to subsequent disease.
According to Belsky et al. 2020 eLife, DunedinPACE is accelerated in young adults exposed to early-life adversity, such as low family social class and higher levels of victimization. We see this same trend in Harden et al. 2021 as well. DunedinPACE is accelerated in children and adolescents exposed to early-life adversity, such as family-level socioeconomic disadvantage and neighborhood-level socioeconomic disadvantage.
To ascertain the potential usefulness of DunedinPACE as a measure for trials of geroprotective treatments, the algorithm was applied to DNA methylation data from a randomized trial of caloric restriction, CALERIE [Ravussin et al., 2015]. The pace of aging clock shows that caloric restriction by 20% does indeed slow the pace of aging in its treatment participants.
In addition, TruDiagnostic (licenseholder of the DunedinPACE algorithm) partnered with Weill Cornell Medicine to perform a longitudinal study of DNA methylation and epigenetic aging in the blood of healthy participants prior to and following test-confirmed COVID-19. The investigation reported, “We also examined whether COVID-19 impacted measures from DNA methylation quantification of the pace of biological aging (DunedinPACE) and observed no significant difference pre vs. post-COVID-19 in these measures.”
DunedinPACE is currently being studied in over 30 clinical trials to understand what really moves this metric.
What is next for this algorithm?
As the cohort from New Zealand continues to age, they will continue to be evaluated for additional insights into the aging process to improve the accuracy and reliability of the DunedinPACE algorithm. However, there are also many more areas for scientific research regarding the benefit of this algorithm.
In a recent press release from TruDiagnostic, Terrie Moffitt from Duke University said:
We are now applying DunedinPACE in 19 other large health-tracking studies. One goal is to test just how sensitively it detects when people change their lifestyles and health behaviors. We are looking at many thousands of people: different ethnic groups, age groups, and men and women, living in different countries. DunedinPACE is the only aging measure so far that was trained on biological change, and the enthusiasm from the international teams who are participating is super exciting!
In addition to looking at the applicability for monitoring age related interventions, this algorithm is being validated in saliva. Investigations into genomic associations have already begun.
If you’re interested in learning the pace of your own aging, measured with the DunedinPACE algorithm, TruDiagnostic currently has the algorithm licensed and available to the public as a report expansion on its TruAge epigenetic testing service.
View the article at lifespan.io