Scientists have discovered a new mechanism of action of one of geroscience’s favorite molecules, which is related to its anti-cancer effect [1].
Rapamycin – more than a geroprotector
Rapamycin needs no introduction in the longevity community. This molecule was discovered in 1972 in a sample of soil from Easter Island (Rapa Nui in the native language), where it was produced by a type of local bacteria. Rapamycin emerged as a potent immunosuppressant and, as such, has been used for decades to dampen immune response in recipients of organ transplants. More recently, rapamycin was approved as a treatment for several types of cancer.
In the 2000s, rapamycin found a new life as a poster child of the longevity field after studies showed that it can significantly increase lifespan in various model organisms, including mice [2]. Rapamycin’s main effect involves the mechanistic target of rapamycin (mTOR), a key player in nutrient sensing. Basically, mTOR gauges the abundance of nutrients and adjusts cellular metabolism accordingly. When mTOR is downregulated, cells act as if nutrients are scarce, dial down their activity, and increase autophagy, the cellular recycling system. At an organismal level, this leads to better health and, hopefully, to lifespan extension. Recently, LEAF crowdfunded the first major study of rapamycin’s geroprotective effects in humans.
Additional targets acquired
Rapamycin does not neatly fit the bill of a caloric restriction mimetic, and scientists have long suspected that it has targets in addition to mTOR. In this new study, the researchers, using novel methods of chemical proteomics, identified STAT3, an oncogenic transcription factor (a protein that regulates other genes’ expression), and its downstream target c-Myc as two new targets of rapamycin.
STAT3 is activated in many cancers and plays a major role in tumor growth via upregulation of anti-apoptotic genes. These genes prevent cells from dying, thus enabling them to proliferate indefinitely and form tumors. One of the targets of STAT3 is c-Myc, an important regulator of cellular metabolism and proliferation. Reflecting the usual level of entanglement we see in biology, c-Myc seems to upregulate STAT3 in a positive feedback loop.
STAT3 and c-Myc are associated with most human cancers, and scientists have been after them for a while [3, 4]. Several methods of STAT3 and c-Myc suppression have been proposed, with moderate success. However, all those strategies were cumbersome and have not been clinically translated due to issues with cell permeability, safety, specificity, or toxicity. One of the problems was that both proteins proved notoriously hard to bind by small molecules.
In this study, the researchers showed that rapamycin prevents STAT3’s translocation to the nucleus (where it can affect expression of other genes) by binding to one of its domains that was previously considered undruggable because of its flat surface area. Moreover, in an unrelated process, rapamycin also directly downregulates c-Myc. Experiments on genetically modified cells with mTOR knocked out showed that rapamycin does all this independently of mTOR, proving that the scientists had indeed discovered a new mechanism of action.
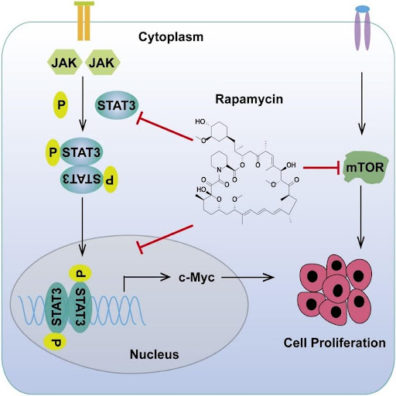
Image: Rapamycin downregulates STAT3, which leads to lower expression of STAT3’s downstream target, c-Myc. Rapamycin also directly downregulates c-Myc in addition to its more widely known anti-mTOR activity. Together, these result in slower cell proliferation, inhibiting tumor growth.
The researchers also ran a few experiments in vivo. In one of them, in mice with induced hepatic tumors, rapamycin significantly decreased tumor size. Levels of mTOR, STAT3 and c-Myc were reduced as well.
Less serendipity, more knowledge
As exciting as this sounds, rapamycin probably does most of its anti-cancer work via the mTOR pathway, since its binding affinity to mTOR is higher than to STAT3. Still, STAT3 and c-Myc are important anti-cancer targets that have proved notoriously hard to approach. The researchers hope that their discovery will open new avenues in engaging these two cancer regulators.
There is also a bigger picture. Although biology has made spectacular progress, scientists are often flying blind due to the immense complexity of biological systems. Even after having serendipitously discovered a compound that does something useful, we rarely know its full mechanism of action and/or full consequences of that action. New high -hroughput approaches in genomics, proteomics, and other -omics can give us a much more nuanced understanding of how things work.
Conclusion
Rapamycin is one of the most promising molecules in geroscience but also an immunosuppressant and an anti-cancer drug. This paper reveals a new mechanism of rapamycin’s action with potentially wide implications. Maybe more importantly, it showcases a method of building a molecule’s targetome by using novel methods of chemical proteomics.
Literature
[1] Sun, L., Yan, Y., Lv, H., Li, J., Wang, Z., Wang, K., … & Zhang, Y. (2021). Rapamycin targets STAT3 and impacts c-Myc to suppress tumor growth. Cell Chemical Biology.
[2] Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., … & Miller, R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. nature, 460(7253), 392-395.
[3] Darnell, J. E. (2005). Validating Stat3 in cancer therapy. Nature medicine, 11(6), 595-596.
[4] Pelengaris, S., & Khan, M. (2003). The many faces of c-MYC. Archives of biochemistry and biophysics, 416(2), 129-136.
View the article at lifespan.io














































