Thank you to ya all with knowledge, I hope you can bear with me (I can be irritating) and help me in this journey (i.e. help answer questions I/noobs have, marking in red): My goal here is to put anti-aging biology in some mental context, specially relating to: a) cell cycle, b) functional tissue cell vs stem cell, c) Telomeres. Though please add more concepts if it can help this understanding.
1. Let us say, we begin our journey with One cell. One cell divides into Two daughter cells. This division process is called Cell Cycle.
2. Cell cycle repeats itself till Hayflick limit of the cell is reached? Is it that when telomere length is left zero, Hayflick limit left = 0 also?
I think before we go further, some basic vocabulary also, if I get straightened:
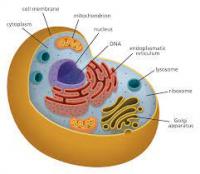
- Cell: has DNA (within Nucleus), Lysosomes/Ribosomes/ER/Mitochondria/Vacuoles etc (within Cytoplasm), Cell membrane
- DNA: important molecule of a living organism.
- Chromatin: is packing structure of DNA (it includes Histones - gene expression proteins)
- Chromosome: are made up of Chromatins (i.e. DNA protein complexes aka Nucleosomes)
- Chromatin changes aka Histone modifications: signify gene expression.
- Telomeres: Protective caps (i.e. Nucleo-protein complexes) at end of Chromosomes. Significance: They mark time?, as they shorten with every cell division.
- Apoptosis: When telomeres run out (besides other reasons too)
- Senescent cell is: when cell has reached its hayflick limit (or got damaged), but did not apoptosed, and continues to hang around?.
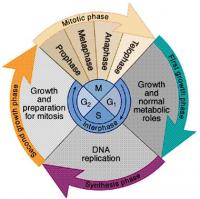
3. The "Cell Cycle"
It seems to me that this cell cycle is for a typical functioning cell only. How do stem cells come/fit into it? Is there some mechanism that first cell tries to fulfill its hayflick potential, and only when it exhausts the cell cycles, that it utilizes a stem cell?
Cell cycle has a broad hoopla of vocabulary: M phase, G1 phase, S phase, G2 phase (skipping all that)
Q1: When people say: Cell differentiation (as in sometimes reported: 'cell did not fully differentiate'), do they mean stem cell differentiation? Since differentiation is concept related to stem cell, as stem cell differentiates into functioning cell A/B/C (where functioning cell can be organ specific, epithelial cell for tissue, cardiomyocyte for heart etc)
Q2: When people say: Cell proliferation. This proliferation concept, I assume is applicable to both a functioning cell (as it will divide till hayflick limit achieved) as well stem cell?
Edited by Learner056, 02 September 2022 - 09:53 PM.