In a robust trial, scientists have shown that NMN supplementation elevates NAD+ levels and increases walking distance in healthy participants, with 600 mg/day being the optimal dose [1].

NMN Increases NAD+ Levels and Fitness in Humans
#1
Posted 13 December 2022 - 04:57 PM
While NAD+ can be supplemented via precursors such as NMN, this route has a few roadblocks as well. For instance, studies do not always show that NAD+ precursors increase NAD+ blood levels [2]. Their safety is a bit of a concern as well, since NAD+ can provide energy to cancer cells via glycolysis [3]. However, in general, NAD+ precursors are currently considered safe.
Robust study design
This study was particularly robust, as it was a randomized, multicenter, double-blind, placebo-controlled clinical trial. ‘Multicenter’ means it was conducted in more than one facility, which helps eliminate possible bias due to facility-specific factors. The primary endpoint was NMN’s ability to elevate NAD+ blood levels. As their secondary endpoints, the scientists chose safety and tolerability, physical performance in a walking test, blood biological age, insulin resistance, and overall health via a subjective assessment.
Previous studies were often inconsistent in showing NMN’s health benefits and its ability to affect NAD+ levels in blood. These studies were often sex-biased and/or conducted in people with pre-existing health conditions. This time, the researchers made a point of recruiting healthy men and women aged 40-65 with a wide range of BMI scores. With 80 participants, the sample size was respectable even if not stellar. Three different doses of NMN were investigated: 300, 600, and 900 mg/day.
This study was co-sponsored by the two companies that jointly produce a food-grade NMN product named AbinoNutra™ NMN. However, it was conducted by a respected team of researchers, so there is no reason to doubt its results. It should also be noted here that NMN supplements were recently banned by the FDA in a controversial ruling.
NAD+ levels and fitness affected
NAD+ levels were increased significantly in all study groups compared to placebo and baseline. There was also a significant difference between 300 and 600 mg/day but not between 600 and 900 mg/day. Interestingly, most of the increase in NAD+ levels happened during the first 30 days of the study, while during the second month, the researchers only saw a very mild additional increase.
The six-minute walking distance at baseline was about 300 meters across the groups. It significantly improved in the three study groups compared to placebo and baseline. Here, too, the difference between 300 and 600 mg/day was large, but the difference between 600 and 900 mg/day was virtually nonexistent. The gains in walking distance were substantial, with both 600 and 900 mg/day groups adding about 150 meters: a 50% increase.
The participants were not required to perform any regular physical activity during the experiment, and the tests were done just three times: at baseline, after the first month, and after the second month. If there was any habituation to the walking test, it should have been noticeable in the placebo group. However, in this group, no increase in walking distance occurred. This means that the 50% increase occurred due to NMN supplementation. It is possible that participants on NMN began feeling more invigorated early into the study period, and increased their physical activity accordingly, which led to better results measured in the clinic.
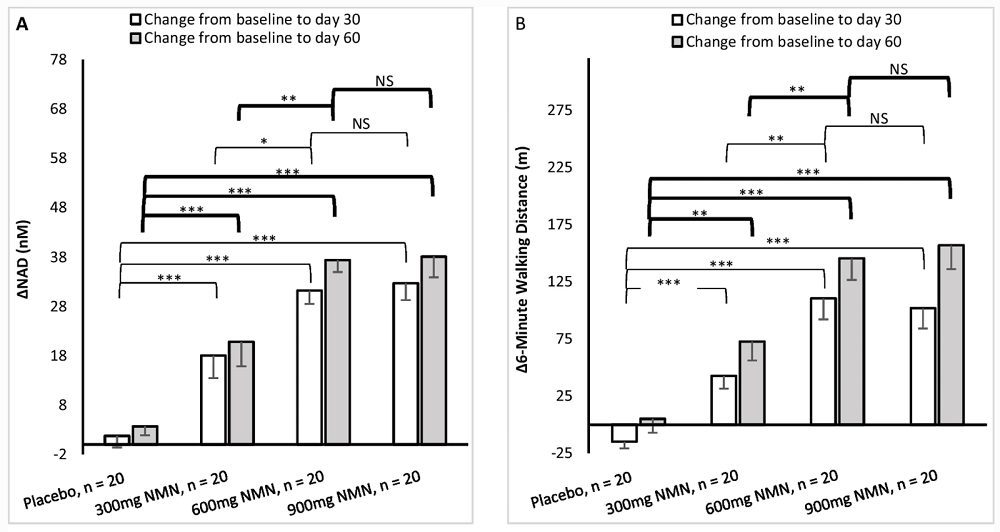
Asterisks above the bars designate different levels of statistical significance, while NS stands for ‘non-significant’.
Puzzling biological age results
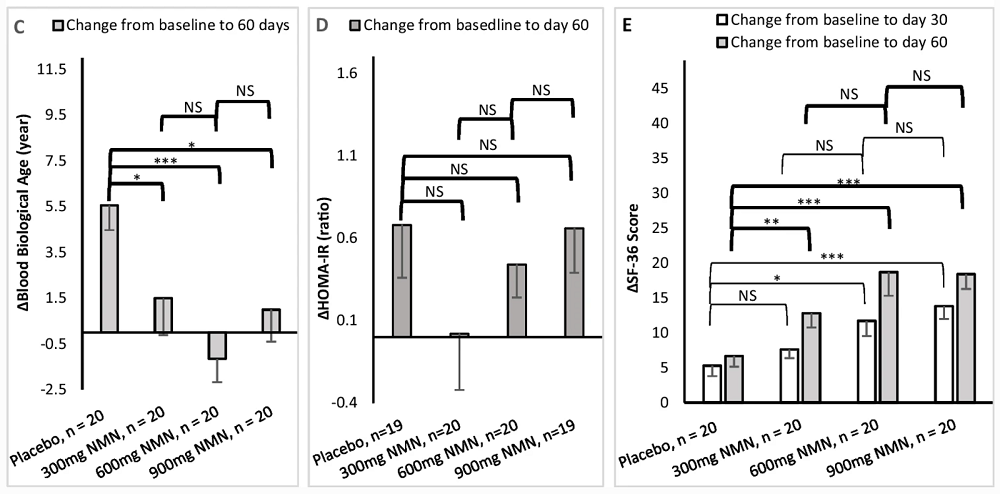
The researchers measured blood biological age using Aging.AI 3.0, which was developed by Alexander Zhavoronkov’s company InSilico. Unlike epigenetic clocks, Aging.AI has yet to see widespread use in scientific studies. According to this metric, in the placebo group, biological age increased by 5.5 years on average from baseline (during the mere two months of the trial’s duration), while in all the study groups, there was barely any change at all. These results look hardly intuitive or interpretable. Unfortunately, the researchers did not use epigenetic clocks that could have provided a benchmark.
For insulin resistance measurements, the researchers used the HOMA-IR (Homeostatic Model Assessment for Insulin Resistance) test. All of those results were statistically insignificant.
The participants were also asked to complete a 36-question health and quality of life questionnaire (SF-36). The scores mostly followed the same dynamic as NAD+ levels and walking distance: there was some increase in the 300 mg/day group compared to placebo (statistically significant at 60 days), and a much more pronounced increase in both the 600 mg/day and 900 mg/day groups. However, there was no significant difference between those two groups.
Conclusion
This interesting trial addresses some design problems found in previous studies and delivers rather robust results, establishing 600 mg/day of NMN as a preferred dose that seems to significantly affect NAD+ levels and physical performance. As in previous NMN trials, no safety problems were reported. However, it does not mean that either efficacy of safety of NMN supplementation have been proven beyond any doubt; therefore, there should be more studies with larger sample sizes, different endpoints, and longer follow-up periods.
Literature
[1] Yi, L., Maier, A. B., Tao, R., Lin, Z., Vaidya, A., Pendse, S., … & Kumbhar, V. (2022). The efficacy and safety of ß-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. GeroScience, 1-15.
[2] Huang, D. (2022). A Multicentre, Randomized, Double Blind, Parallel Design, Placebo Controlled Study to Evaluate the Efficacy and Safety of Uthever (NMN Supplement), an Orally Administered Supplementation in Middle Aged and Older Adults. Frontiers in Aging, 26.
[3] Yaku, K., Okabe, K., Hikosaka, K., & Nakagawa, T. (2018). NAD metabolism in cancer therapeutics. Frontiers in oncology, 8, 622.
View the article at lifespan.io