A new publication in Nature Aging has explained a great deal about aging of the neurovascular system, showing where and how the brain’s blood supply changes with aging in a mouse model.
A branching network of blood vessels
In the neurovascular system, arteries lead to arterioles, which then branch off into precapillary sphincters. These lead to first-order capillaries, then second-order capillaries, and so on, and these microscopic tubes give oxygen and nutrients to every cell in the brain. Previous research has found the arteriole-capillary junction to be significant in regulating how well these vessels deliver their contents (perfusion) [1]. Unfortunately, capillary cells known as pericytes fail with age, thus leading to neurovascular damage [2].
The researchers in this study set out to better understand how per interact. With mice genetically engineered to express fluorescent proteins in pericytes and other vascular cells (mural cells), the researchers used advanced imaging to determine the tiny neurovascular differences between young and old animals.
Dilation and constriction
Using two-photon microscopy, the researchers examined the capillaries of anaesthetized mice, using electrical stimulation to dilate the vessels. They found that the amount of this dilation was significantly decreased with age in both amount and duration, although second-order and smaller capillaries did not dilate significantly more. However, the onset time and excitatory potentials were found to be unaffected. This suggests an increase in vascular stiffness rather than electrical changes in the brain.
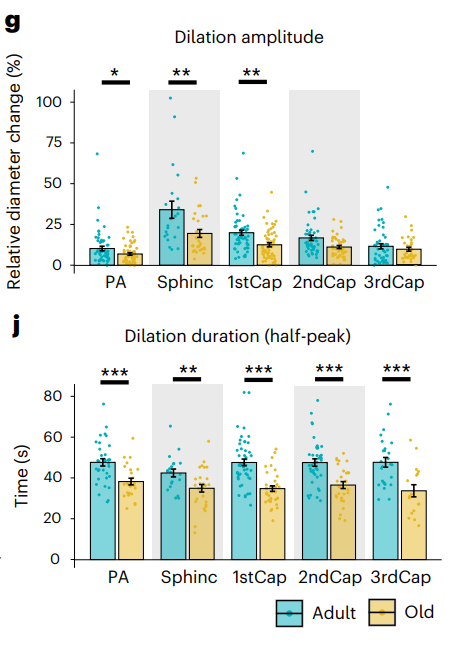
The researchers looked deeper into the causes of this stiffness. Using pinacidil and papaverine, two drugs that encourage dilation through their actions on ions, the researchers surmised that the only remaining restraints on dilation were physical ones, namely extracellular matrix elements such as elastin and collagen.
The next step was to test the opposite of dilation: constriction. Using the well-known vasoconstrictor endothelin, the researchers found that constriction ability was preserved everywhere but the precapillary sphincter.
A decline in density
The researchers then carefully examined the brains of the mice. While the number of mural cells did not decline with age, the density of nuclei did, and these cells covered less of the surface area of the capillaries; this occurred everywhere except the precapillary sphincter. This loss of coverage was found to be linearly correlated with an increase in blood pressure.
This was matched by changes in these small vessels’ size. Aged mouse brains had shorter but wider capillaries than their younger counterparts, although this did not apply to the smallest vessels. The resesearchers hypothesize that this increase in width, which also increases flow, is how old brains compensate for their reduced ability to dilate and constrict. It was also noted that the veins responded by increasing in volume and increasing the total vascular volume of the brain. Additionally, arterioles became more curved and twisted (tortuous) with age.
Abstract
The microvascular infow tract, comprising the penetrating arterioles, precapillary sphincters and frst-order capillaries, is the bottleneck for brain blood fow and energy supply. Exactly how aging alters the structure and function of the microvascular infow tract remains unclear. By in vivo fourdimensional two-photon imaging, we reveal an age-dependent decrease in vaso-responsivity accompanied by a decrease in vessel density close to the arterioles and loss of vascular mural cell processes, although the number of mural cell somas and their alpha smooth muscle actin density were preserved. The age-related reduction in vascular reactivity was mostly pronounced at precapillary sphincters, highlighting their crucial role in capillary blood fow regulation. Mathematical modeling revealed impaired pressure and fow control in aged mice during vasoconstriction. Interventions that preserve dynamics of cerebral blood vessels may ameliorate age-related decreases in blood fow and prevent brain frailty
Conclusion
This paper is rich with information about the potential causes and effects of mural cells, arterial aging, and the fundamental processes involved. Most notably, it points towards a potentially strong effect of extracellular matrix stiffening, a topic that is well-known in the world of geroscience.
Literature
[1] Zambach, S. A., Cai, C., Helms, H. C. C., Hald, B. O., Dong, Y., Fordsmann, J. C., … & Lauritzen, M. J. (2021). Precapillary sphincters and pericytes at first-order capillaries as key regulators for brain capillary perfusion. Proceedings of the National Academy of Sciences, 118(26), e2023749118.
[2] Bell, R. D., Winkler, E. A., Sagare, A. P., Singh, I., LaRue, B., Deane, R., & Zlokovic, B. V. (2010). Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron, 68(3), 409-427.
View the article at lifespan.io









































