Scientists have shown that extracellular vesicles derived from senescent stem cells can improve the proliferation, viability, and migration capacity of healthy stem cells [1].
Cell-to-cell packages
Extracellular vesicles (EVs, not to be confused with electric vehicles) are tiny membrane-bound bubbles that are emitted by cells and carry various molecular cargo such as proteins and miRNA. EVs have been gaining recognition as an important tool of intercellular communication [2] that can trigger functional changes in the receiving cell. EVs are also highly indicative of the emitting cell’s health, which has led to a recent proposal to recognize them as a new hallmark of aging.
EVs emitted by senescent cells have been implicated in inducing senescence in the receiving cells (paracrine senescence) [3]. However, as this new study suggests, such EVs can also have a positive effect.
Increased antioxidant production
The researchers experimented on stem cells taken from human dental pulp. In the body, those cells reside in a low-oxygen environment (3%-6%). Placing them under room conditions (21% oxygen) results in severe oxidative stress, which drives senescence, as this same group of researchers demonstrated in an earlier study [4].
Oxidative stress conditions led to an increase in EV production. It has been hypothesized that EVs also serve as a method of “waste disposal” excreting harmful molecules when the cell’s inner recycling machinery is overwhelmed, such as when the cell is under a lot of stress [5].
The researchers isolated EVs from the senescent cells. They then co-cultured those EVs with healthy young dental pulp stem cells kept under favorable conditions (3% oxygen) and compared mRNA levels of three major antioxidant enzymes – superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) – in the senescent cells, healthy stem cells infused with EVs derived from senescent cells, and healthy controls.
The production of those antioxidants was increased in the senescent cells, apparently to counteract oxidative stress. Interestingly, EVs derived from those senescent cells caused a significant upregulation of those molecules in healthy cells.
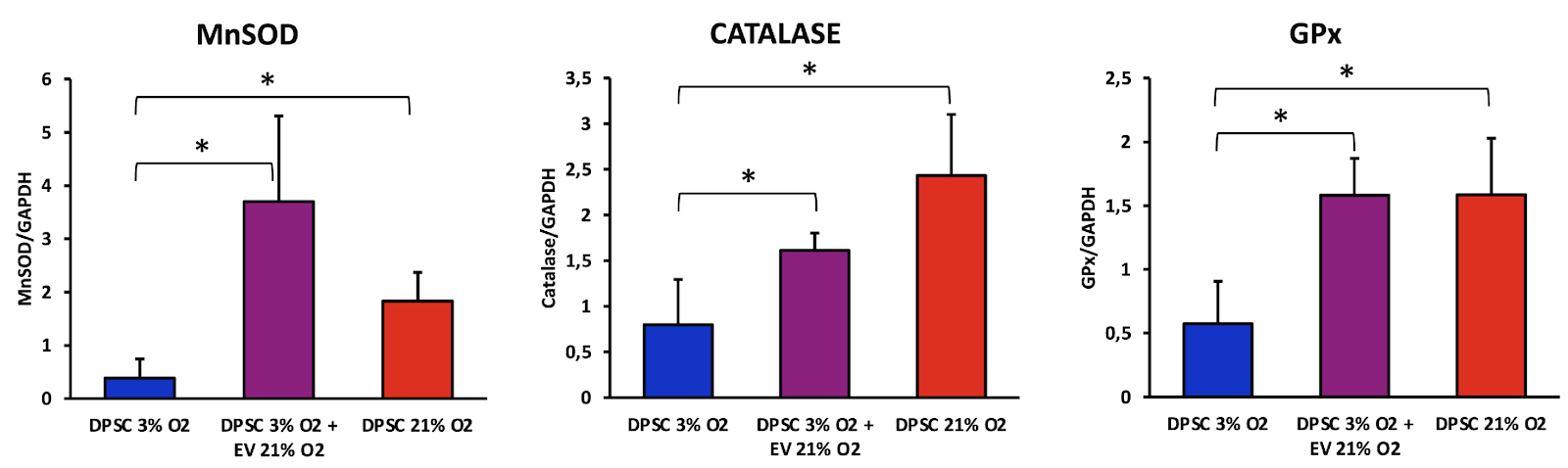
Altered mitochondrial function
Senescence-associated EVs also caused changes in mitochondrial function in the recipient cells. This included an increase in basal respiration and a decrease in maximal respiration. The difference between those two is called the spare or reserve bioenergetic capacity and is generally indicative of mitochondrial health, although this is context-dependent.
The researchers interpreted this as the recipient cells slowing down their mitochondrial bioenergetic flow to reduce the intracellular production of reactive oxygen species (ROS). In other words, cells “thought” they were already in an oxidant environment and tried producing less ROS to not exacerbate the situation.
The researchers suggest that this hypothesis is supported by the fact that the treatment did not significantly change mitochondrial membrane potential and the levels of mitochondrial peroxides, indicating preserved mitochondrial health and function, which had declined in the senescent cells.
EVs boost healthy cells’ fitness
Interestingly, the EV treatment did seem to increase the recipient cells’ fitness. While proliferation and viability were impaired in the senescent cells, EVs caused the opposite effect in healthy cells. The treatment also highly significantly decreased apoptosis (cell death) levels even compared to healthy controls.
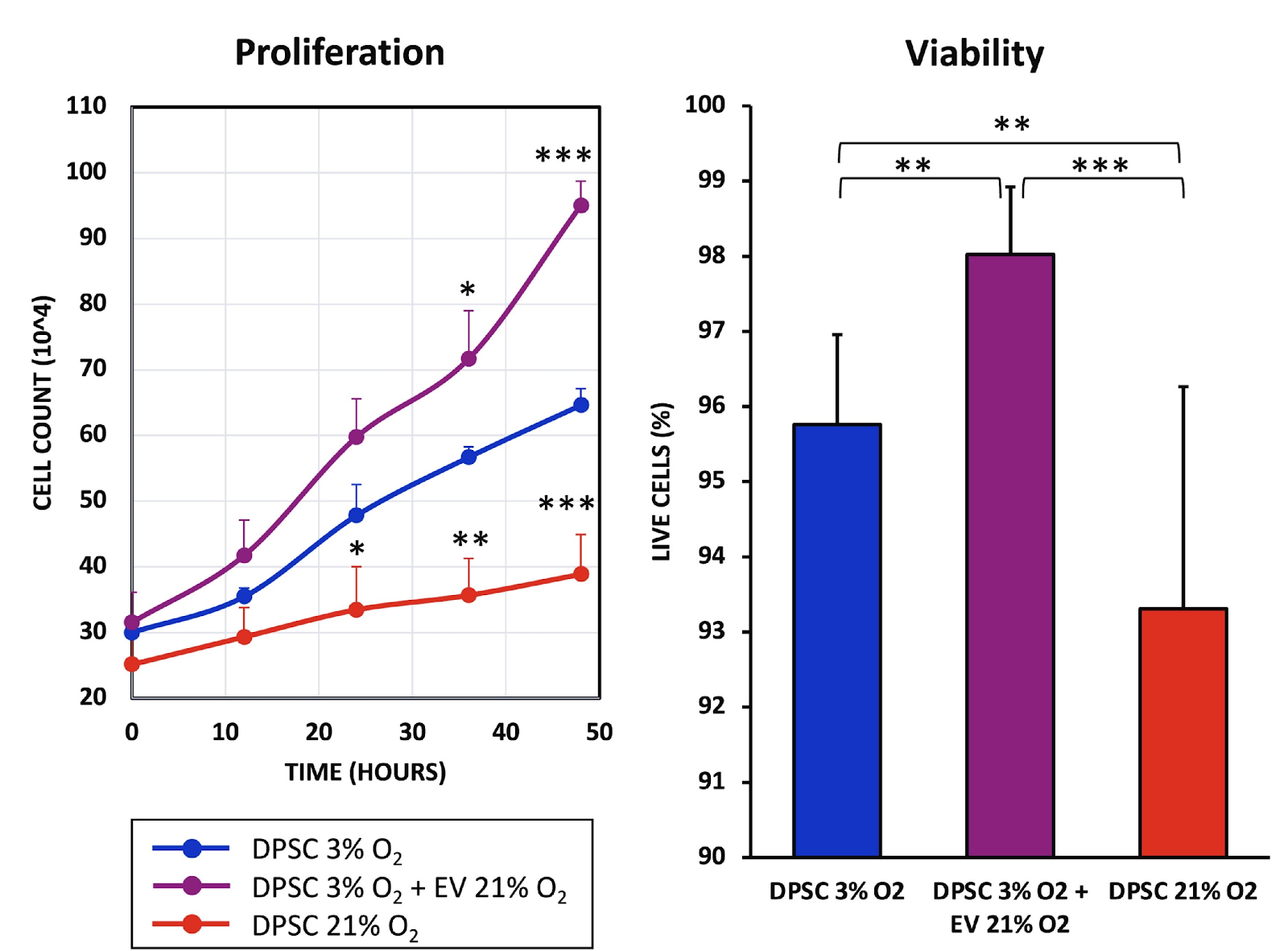
The same dynamic was observed when the researchers tested the cells’ migration capacity in a wound-healing assay. The EV-treated stem cells closed the gap faster than healthy controls, which, in turn, did this much faster than the senescent cells. The beneficial effect of senescent cells in wound healing is well known, and the researchers suggest that conditioning other cells via EV transfer might be one of the mechanisms involved.
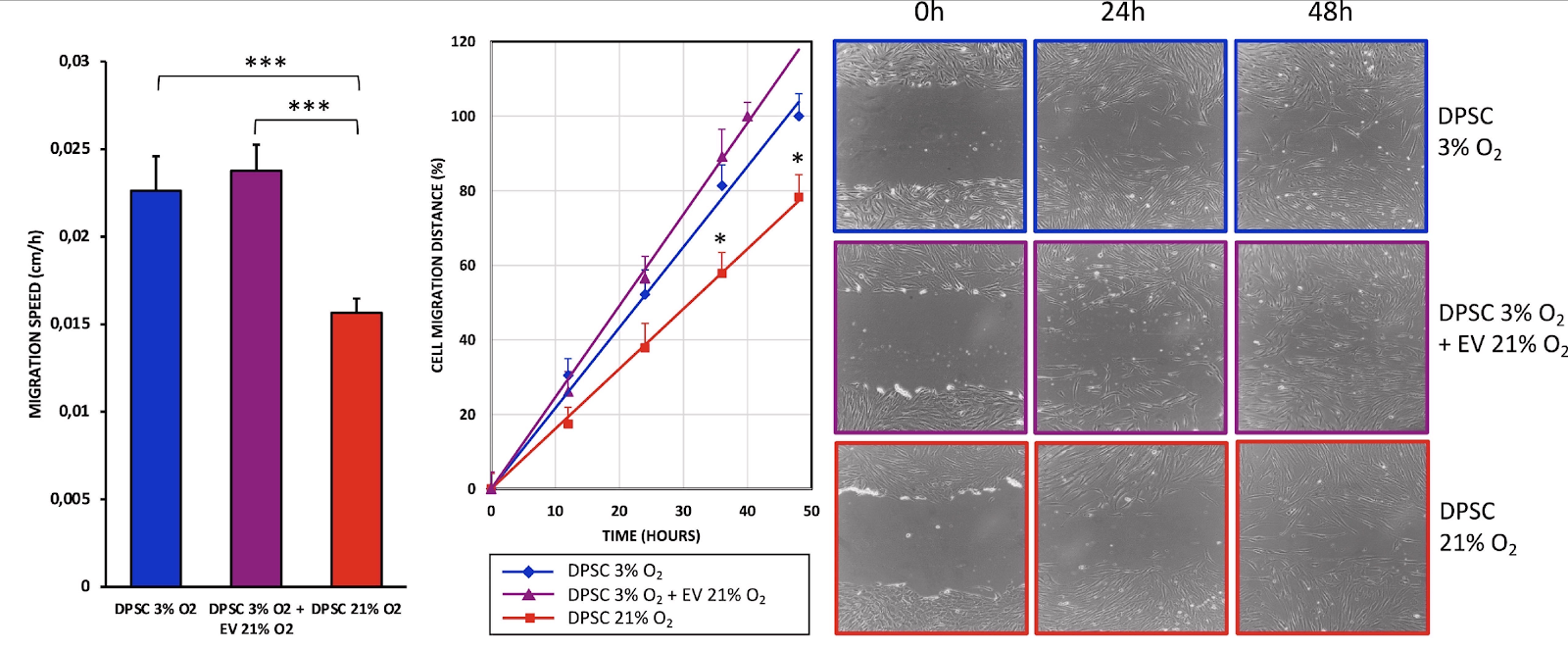
The results show that extracellular vesicles from senescent stem cells induce overexpression of antioxidant genes (MnSOD, CAT, and GPx) in young stem cells, which show an increased non-mitochondrial oxygen consumption, accompanied by reduced maximal respiration and spare respiratory capacity without altering mitochondrial membrane potential. This is accompanied by improved cell proliferation, viability, and migration rates and a reduction of apoptosis. In conclusion, extracellular vesicles from senescent stem cells trigger an adaptive response in young stem cells which improves their antioxidant defenses and their proliferation, migration, and survival rates. This suggests that extracellular vesicles can modulate the cells’ microenvironment and the balance between proliferation and senescence.
Conclusion
Cellular senescence is a phenomenon that is central to aging but also has multiple other effects. This study’s intriguing results demonstrate the effects of “conditioning” healthy cells by EVs emitted by senescent cells, which might partially explain their role in wound healing and tissue remodeling. This could possibly be utilized to “supercharge” stem cells for cancer therapy and other uses.









































