Today, we bring you a selection of presentations from the annual conference organized by the Gerontological Society of America.
Most of our savvy readers, if asked to name the biggest gerontology conference, would probably go with the venerable ARDD in Copenhagen or the new favorite, Hevolution Foundation’s Healthspan Summit in Riyadh. Yet, both pale in comparison with the enormous conference (still not the biggest in the world though) organized annually by the Gerontological Society of America. GSA 2024 was held in my hometown of Seattle earlier this month, and I was there to witness all the grandeur.
And big it was! There were 4,000 participants over four days (five if you count the pre-conference workshops), and it occupied the entire four floors of the Seattle Convention Center. However, the lion’s share of the conference was focused not on the biology of aging or longevity biotech companies but rather on caring for the elderly and the societal aspects of aging. We in the longevity field tend to forget about this immense industry of helping people during their last decades of life and the legions of dedicated people who provide this help.
In sharp contrast, the biology of aging track at the conference was tiny, with a couple of dozen people struggling to fill the modestly sized auditorium. Nevertheless, it featured high-quality talks and some of the biggest names in the longevity field, such as Harvard Professor David Sinclair. Sinclair’s talk was a part of the pre-conference workshop on the hallmarks of aging, which makes it a great starting point for this selection of talks.
Cellular reprogramming is moving closer to the clinic
Fame and controversy notwithstanding, Sinclair remains one of the leading and most productive geroscientists, conducting cutting-edge research out of his Harvard lab and overseeing a few companies. In his talk, he provided an overview of his information theory of aging and the recent research by his team, which we have previously featured on our site.
Information, Sinclair said, is becoming ever more relevant to understanding aging. All the hallmarks of aging “talk to each other.” However, with time, this biological information becomes corrupted by “noise,” which is introduced in several ways, including via DNA mutations and unwanted changes in gene expression (epimutations).
Mutations and epimutations are inextricably linked by the fact that certain proteins (namely, some of the members of the sirtuin family) participate both in regulating gene expression by repressing chromatin and in repairing DNA breaks. When those proteins are summoned to a DNA break, they abandon their posts as chromatin guardians and do not always successfully return.
Sinclair’s lab has created a mouse model involving induced changes to the epigenome (ICE) into which the researchers can induce a modest amount of DNA breaks, which are faithfully repaired, thus not leading to mutations – however, the repair process leads to epimutations. This allowed the team to uncouple genetic and epigenetic changes and look at how the latter affect aging. According to Sinclair, experiments with ICE mice have confirmed that epigenetic changes induce aging across multiple hallmarks, even in the absence of DNA mutations.
Sinclair’s idea is that the information required to restore the epigenome to its original or at least younger state, which happens during cellular reprogramming, must be stored somewhere in the cell; therefore, every epigenetic change is “recorded” by yet unknown molecular mechanisms. Sinclair believes his group might have taken the first steps in identifying these mechanisms: they showed that the enzymes SIRT1 and TET2, which are involved in epigenetic alterations, bind to the same sites in certain genes as the Yamanaka reprogramming factors.
Another direction that Sinclair pursues is cellular reprogramming’s clinical applications. His team has developed a protocol that uses three of the four classic Yamanaka factors (OCT4, SOX2, and KLF4, or “OSK”), deliberately omitting c-Myc, which is an oncogene. In addition to improving safety, this allows for continuous expression of the reprogramming factors without throwing the cells back into pluripotency. For more details, read our recent interview with Sharon Rosenzweig-Lipson, CSO of Life Biosciences, one of Sinclair’s companies.
Life builds on Sinclair’s work in restoring vision via cellular reprogramming. In his talk, Sinclair showed a video of a formerly blind aged mouse responding to visual stimuli after just three weeks of OSK treatment. Experiments have also been performed on rats and non-human primates. Life Biosciences might be very close to bringing cellular reprogramming to the clinic, as Sinclair announced human trials scheduled for August 2025.
While gene therapy with these factors would be expensive, the Sinclair lab is developing more affordable alternatives. The researchers have identified cocktails of small molecules that can induce partial cellular reprogramming.
The research has progressed from cells to miniature tissue models (organoids). The researchers’ work with cellular senescence has delivered particularly promising results, with senescent cells showing dramatic improvements after just ten days of treatment.
While the initial focus is on eye diseases such as glaucoma and AMD (age-related macular degeneration, a very hard disease to tackle), Sinclair hopes that their findings “might be relevant to a whole variety of diseases, not just the eye.”
From toast to aging: the hidden impact of glycation
For most of his career, Prof. Pankaj Kapahi of the Buck Institute on Aging has been studying glycation, a non-enzymatic chemical reaction where sugar molecules, such as glucose, bond to proteins, lipids, or DNA. Glycation is also the chemical process that makes toast brown and flavorful (“We’re slowly toasting away,” Kapahi joked).
Glycation impairs molecules’ normal function and is known to contribute to aging and diseases like diabetes and Alzheimer’s. Advanced glycation end-products are abbreviated as AGEs.
Kapahi’s recent research focuses on methylglyoxal (MGO), a highly reactive molecule produced whenever cells use glucose and a precursor to AGEs. “It’s about 1,000 times more reactive than glucose,” Kapahi explained. “It has both aldehyde and ketone groups that can bind DNA, proteins, and lipids through covalent bonds, and there’s no escape from its production.”
This reactivity affects multiple hallmarks of aging, including mitochondrial function and epigenetic state. The researchers discovered that it also drives cellular senescence. Things are complicated by the fact that MGO might not be just a harmful byproduct but also have regulatory roles, including appetite regulation and glycolysis control.
“Our current research shows glycation accelerates aging in multiple organs: heart, eye, fat cells, brain (by affecting myelination), and pancreas,” Kapahi said. “It also increases senescence markers in fat and affects glucose homeostasis.”
The team screened about 600 compounds to find AGE-lowering agents. Bictinamide emerged as a promising candidate, and the team has developed a five-compound cocktail. It includes lipoic acid, which effectively reduces the burden of methylglyoxal and AGE across tissues.
This cocktail, called Gly-Low, improves aortic stiffening, glucose tolerance even in normal mice, and neuromuscular balance. Impressively, it extends lifespan in mice when administered late in life (around 24 months). In high-fat diet models, it reduces blood glucose and improves glucose tolerance while significantly reducing inflammatory cytokine loads.
The researchers are now investigating the mechanisms behind these effects. “We’re using click chemistry to identify methylglyoxal-modified proteins,” Kapahi explained, “hypothesizing that these modifications might make proteins appear foreign to the immune system, triggering inflammatory responses. This work should help us understand how glycation drives aging and age-related diseases.”
When life gives you fibroblasts, make neurons!
Larissa Traxler from Jerome Mertens’s Lab at UC San Diego gave a talk titled “From Old Skin to Old Neurons: Direct Conversion to Explore the Interface between Cellular Aging and Disease.”
One of the reasons we lack cures for diseases like Alzheimer’s, she said, is because aging, a major risk factor, is so heterogeneous. No unifying etiology for Alzheimer’s exists, and almost all cases are sporadic. This inspired Traxler’s team to develop individual-specific analysis approaches that can capture this heterogeneity, which they achieve by using direct cellular reprogramming of patient-derived fibroblasts into neurons.
Starting from patient skin biopsies, they then use lentiviral factors to induce direct conversion with 40-50% efficiency. After cell sorting, more than 95% purity is achieved. The resulting induced neurons (iNs) are a combination of excitatory and inhibitory types and are mostly similar to frontal cortex neurons. Using various combinations of transcription factors such as Ngn2 and Ascl1, the team can generate different neuronal subtypes.
A crucial aspect of the system is that these iNs maintain the biological age of the donor and their specific aging signature. “This is fundamentally different from iPSC-derived neurons, which reset to pre-birth ages according to methylation clocks,” Traxler said. “Our iNs reflect individual donor ages and display adult-stage characteristics, while iPSC neurons resemble fetal stages.”
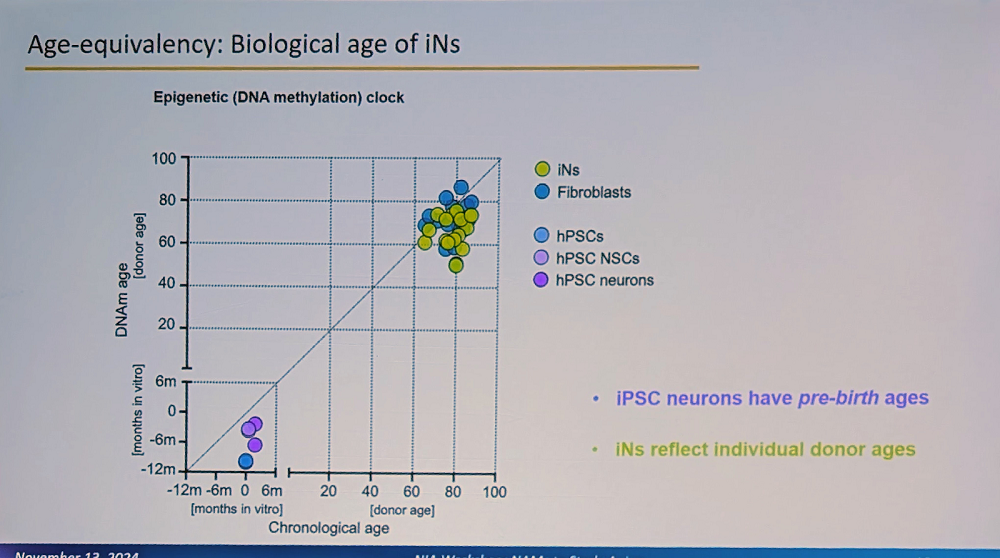
This age retention is particularly visible in adult neuronal splicing, such as in MAPT (Tau) genes, where the researchers observe adult-specific combinations of isoforms and their phosphorylation. Interestingly, humans have much more alternative splicing than mice, a feature that cannot be properly modeled in mouse systems (mouse models of Alzheimer’s have indeed been unreliable, and their relevance to human Alzheimer’s is questioned).
The group went even further, having developed multicellular constructs that combine iNs with glial cells on polymer scaffolds. These form dense three-dimensional structures with synaptic connections. In these 3D constructs, the researchers have observed amyloid deposition with clear differences between familial and sporadic AD cases.
Interestingly, we recently reported on a study claiming that low efficiency of direct fibroblast-to-neuron reprogramming happens because only a small subset of stemlike cells (neuron crest progenitor cells) embedded within differentiated skin cells can produce neurons. However, the much higher efficiency cited by Traxler seems to contradict this claim. It is possible that her team uses a stronger reprogramming protocol that can cause a wider variety of skin cells to transition into neurons.
Can this diabetes drug extend lifespan?
Carolina Solis-Herrera from the University of Texas spoke at a session focused on repurposing existing drugs for slowing aging. Sodium-glucose cotransporter-2 (SGLT2) inhibitors, originally developed as glucose-lowering medications for type 2 diabetes, have attracted considerable attention in the longevity field after evidence appeared that this class of drugs positively affects both lifespan and healthspan. In particular, canagliflozin was among the handful of drugs that produced significant life extensions in mice in the rigorous Interventions Testing Program (ITP) trials.
Solis-Herrera’s group is trying to unravel the mechanisms of action behind those benefits. Recent evidence from trials shows that patients on SGLT2 inhibitors, both with and without diabetes, experienced fewer cardiovascular events, reduced hospitalization for heart failure, and improved kidney function. Cardiovascular and renal problems are, of course, two major causes of death in both diabetic and non-diabetic people.
“The cardiovascular protection we see with SGLT2 inhibitors emerges remarkably quickly – between six to eight weeks, far too fast to be explained by traditional risk factor improvements,” Solis-Herrera said. “This suggests there must be other mechanisms at work.”
Results show that SGLT2 inhibitors work along several pathways. They enhance the clearance of senescent cells; induce calorie loss by promoting urinary glucose excretion, which resembles caloric restriction; modulate key nutrient-sensing pathways involved in aging, such as mTOR and AMPK; and reduce age-related low-grade inflammation (inflammaging) and oxidative stress.
The researchers currently focus on the “ketone hypothesis”: that SGLT2 inhibitors increase the production of ketone bodies, such as beta-hydroxybutyrate (BHB). “We found that ketones reduce oxygen consumption and increase efficiency in various organs, including heart and kidneys,” Solis-Herrera said. “We’re now investigating applications in Alzheimer’s, diabetic retinopathy, dementia, and post-transplant patients for reducing rejection – that’s why we call SGLT2 inhibitors “the gift that keeps giving.”
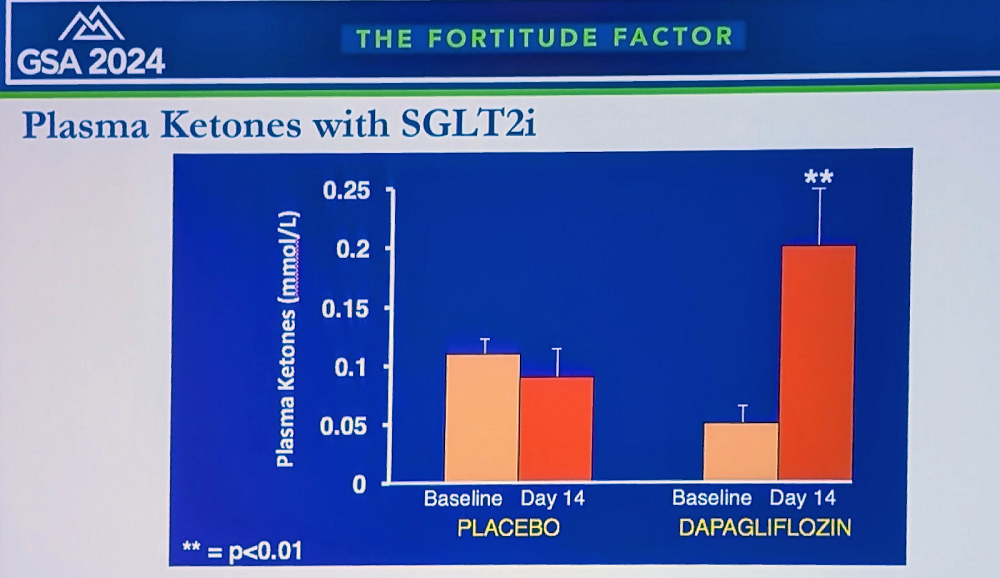
Solis-Herrera reported on a new study in humans, where patients on SGLT2 inhibitors showed decreased inflammatory biomarkers, including TNF-alpha; a decrease in oxidation markers and senescent cell burden; and a significant decrease in visceral fat across multiple organs. “In summary,” she concluded, “SGLT2 inhibitors, originally created for diabetes, have emerged as potentially powerful anti-aging compounds. Their mechanisms likely involve a metabolic shift from glucose excretion to ketogenesis, which appears to be beneficial rather than maladaptive. They modulate key molecular pathways like mTOR and AMPK, reducing inflammation and oxidation.”
Looking beyond weight loss
John Newman, another representative of Buck Institute on Aging at the conference, talked about an even more hyped type of drug: glucagon-like peptide (GLP-1) receptor agonists, such as semaglutide, the principal ingredient of Ozempic and Wegovy. These drugs have revolutionized the treatment of diabetes and obesity, but many researchers believe they provide anti-aging benefits outside this context. Newman said he was very enthusiastic about GLP-1 agonists, but “there are critical gaps that need filling before we let that enthusiasm run away.”
Newman explained that GLP-1, a small peptide hormone secreted by L cells in the gut epithelium and circulating throughout the body, reduces motility in the gut and slows glucose absorption. In the pancreas, it enhances glucose-stimulated insulin secretion to reduce hyperglycemia. However, endogenous GLP-1 is rapidly degraded, with a half-life in plasma “in the order of minutes.”
The GLP-1 receptor also interacts intracellularly with various aging-related pathways, such as mTOR and FOXO, enhancing mitochondrial function and dampening inflammation. “All these pathways,” Newman said, “are very familiar to geroscientists, and this integration of GLP-1 receptor signaling with mechanisms of aging is part of why the idea of GLP-1 receptor agonists as gerotherapeutics is so tempting.”
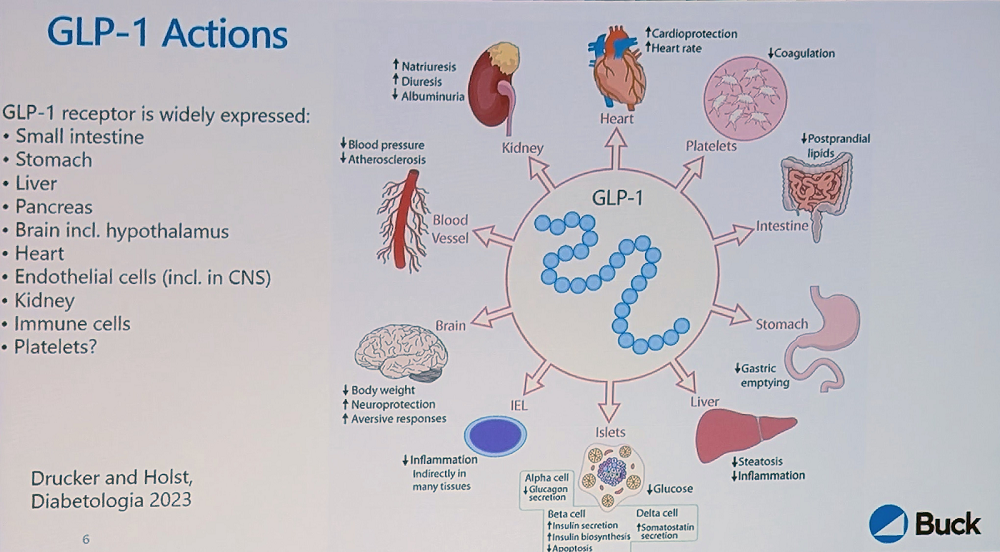
The GLP-1 receptor is widely distributed in the body, including in hypothalamic neurons, which is how it regulates satiety and appetite. It is also present in the heart, which might explain the cardiovascular benefits.
Large-scale trials have shown a significant decrease in cardiovascular mortality in obese and/or diabetic patients. However, gastrointestinal side effects are a major problem: virtually all patients on GLP-1 receptor agonists will eventually experience them, and they are severe enough to cause a 5%-8% dropout in studies.
“The results are striking for diabetes treatment and, within either diabetes or obesity, for atherosclerotic disease, kidney disease, and heart failure with preserved ejection fraction,” Newman said. “But does this make them gerotherapeutics? Not necessarily – it makes them highly effective diabetes and obesity treatments.”
For us to decide that these drugs are indeed geroprotectors, he explained, we have to see efficacy in diseases outside this metabolic cluster, such as neurodegenerative disease, cancer, and osteoporosis. Small trials in Parkinson’s and Alzheimer’s diseases showed some promise, but the results were not dramatic. A large Alzheimer’s trial (EVOKE) is ongoing. Importantly, this is one of the first large trials in non-obese people.
“The big picture,” Newman summarized, “is that while these are very effective agents in obesity and diabetes, we don’t know if benefits extend beyond these conditions or if cardiovascular and kidney protection is independent of weight loss – crucial questions for their potential as gerotherapeutics. Questions remain about the effects on sarcopenic obesity and diseases of aging not caused by obesity or diabetes. Much work remains to be done.”
Cellular reprogramming for organ transplantation
Pradeep Reddy of Juan Carlos Izpisua Belmonte’s lab gave a fascinating talk on cellular reprogramming. Belmonte is one of the pioneers of partial reprogramming and the first to demonstrate significant life extension in progeroid mice. Like several other first-tier geroscientists, Belmonte, previously at Salk Institute, was recruited by Altos Labs. Reddy’s talk presented a rare opportunity to gauge how things are going at the best-funded longevity startup in the world.
Reddy started by bringing up the lab’s work from several years ago on Hutchinson-Gilford progeria syndrome (HGPS). When the researchers reprogrammed fibroblasts from HGPS patients to induced pluripotent stem cells (iPSCs), “one striking observation was that all the aging hallmarks their cells initially showed were totally reversed, even though the mutation was still present,” Reddy said.
When these iPSCs were re-differentiated back to somatic lineages, they started to manifest the disease phenotype again. However, the team realized the importance of the first part: “that it’s possible to take a pre-diseased cell and reset or reverse those disease markers.”
Previously, partial reprogramming was mostly discussed in the context of aging. However, their results led the team to look for opportunities to apply reprogramming to contexts other than aging, “changing the trajectory of cells from diseased to healthy.”
“We conducted several studies in aged animals across different tissues,” Reddy said. “It’s not specific to only certain cell types – it can be a broad, agnostic approach. One thing that happens during loss of chromatin stability in disease or aging is loss of cell identity, which leads to decreased functional fidelity.”
One epigenetic alteration that is ubiquitous and important in aging is the epithelial-to-mesenchymal transition (EMT), in which epithelial cells lose cellular adhesion and become more motile. EMT can play a beneficial role in wound healing, but it also harms the original function of epithelial cells and is one of the central mechanisms of invasion and metastasizing in cancer.
The researchers observed increased EMT signatures in models of liver disease. “Similarly, we see the same phenotype in different cardiomyopathies, lung, and kidney disease,” Reddy said. “It’s a common phenotype, not specific to one tissue.” Partial reprogramming led to the erasure of these mesenchymal signatures in a matter of two to four days, which can explain early benefits.
One area where the researchers attempted to apply partial reprogramming is cellular senescence since senescence cells undergo drastic epigenetic changes. They saw decreased levels of SASP (senescence-associated secretory phenotype) elements such as p16, increased resilience, less hair graying, and improved wound healing in treated mice.
Delivering reprogramming factors via viral vectors remains a challenge since, with systemic delivery, most particles end up in the liver. Reddy’s team sought to rejuvenate kidneys, but the delivery problem seemed insurmountable until they decided to take a page from clinical practices, where donor organs are often connected to perfusion machines ex vivo for up to several hours to keep them viable. The idea was to add reprogramming factors to the perfusion solution in order to increase the organ’s fitness.
In collaboration with a clinic in Barcelona, the researchers worked on kidney transplantation in rats. As expected, organs from old donors were less viable, but reprogramming during perfusion showed promising results.
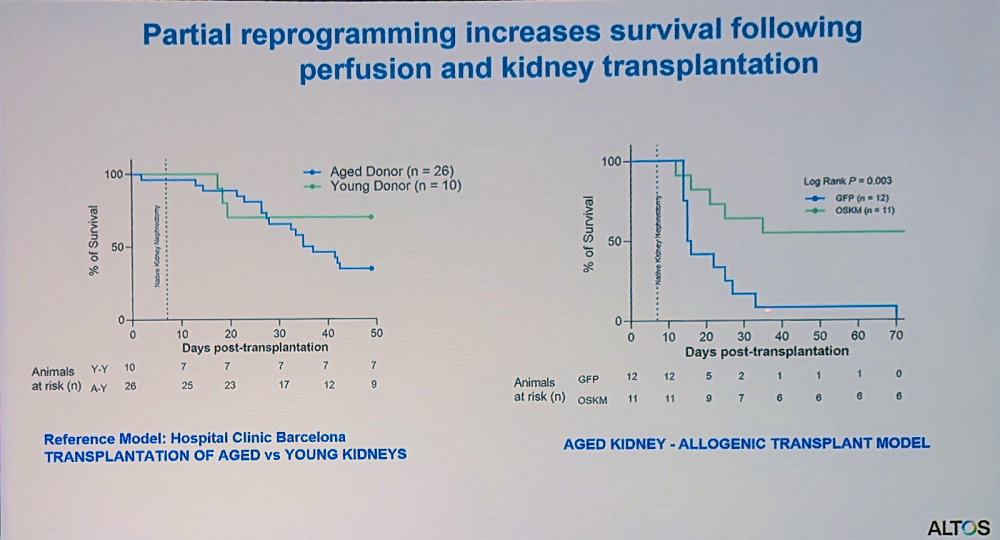
“This platform could help expand the donor pool by allowing us to improve suboptimal organs that would otherwise be discarded,” Reddy said. “We’ve built a modified perfusion system that doesn’t require complete transplantation – organs can remain connected to the body while undergoing perfusion, making it more applicable for age-related disease cases.”
No limit for New Limit
Not as hyped as Altos Labs, New Limit is nevertheless another exciting company working in the field of cellular reprogramming. Jacob Kimmel, who co-founded the company after stints at UCSF and Alphabet’s anti-aging company Calico, gave a captivating overview of its research.
New Limit is built on the premise that the classic Yamanaka reprogramming factors (OSKM) are not special, and many other rejuvenating factors and combinations can be found, tailored to specific cell types and contexts. “We plan to formulate these combinations into medicines using mRNA technology, similar to what many of us experienced with COVID vaccines,” Kimmel said. “Finally, we want to deploy these clinically to treat pathologies that will eventually affect all of us.”
While New Limit is working on several cell types and indications, in this talk, Kimmel focused on their T cell program, which targets infectious diseases by improving resilience. “There’s an enormous number of possible combinations,” he said. “We can’t experiment our way through that, so we need to be both efficient and smart about which experiments we choose.”
To solve this problem, New Limit has developed a proprietary high-throughput discovery process that begins with predictive computer models. The work then moves into primary human cells from multiple young and old donors.
When the researchers introduce pools of transcription factors, “due to the stochasticity of delivery, each cell picks up a different subset of factors,” Kimmel explained. “The result is a dish where all possible subcombinations up to a certain number are represented. We’ve attached DNA barcodes to these factors, allowing us to use single-cell genomics downstream to measure what happened – which genes the cell is expressing and which transcription factors achieved that outcome.”
To detect if any combination resulted in rejuvenation, the researchers use machine learning models to predict cell age from gene expression profiles. Using this system, they have screened around 9,000 combinations of transcription factors for their effect on cell age – “about 500 times more than the roughly 19 combinations tested in academic literature,” Kimmel said.
Interestingly, the researchers have found that many different transcription factors can reverse T cell aging, often to the same degree as the Yamanaka factors. That said, those factors were not just variants of Yamanaka’s but are “broadly distributed across different transcription factor families, suggesting multiple paths to reprogram cell age.” Kimmel reported seeing a lot of synergy as if transcription factors tend to work better in combinations.
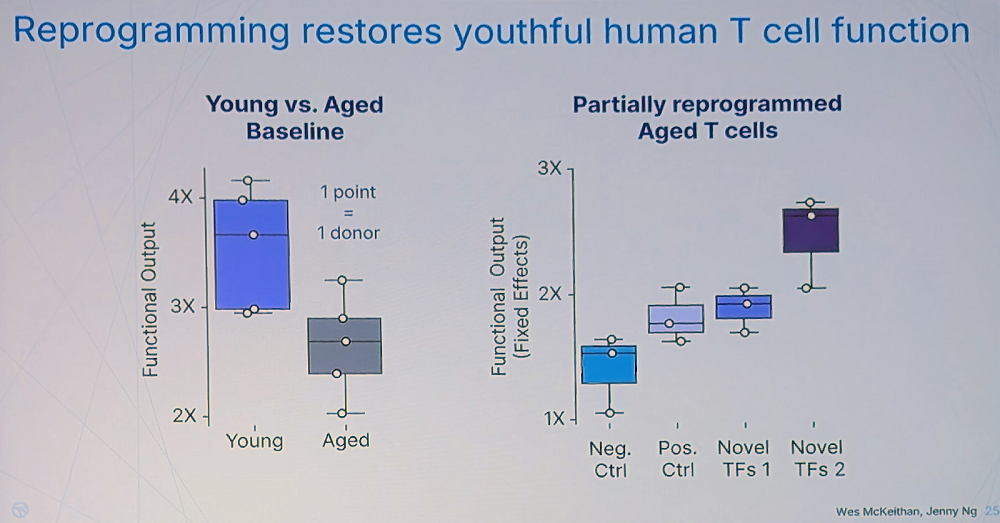
Rejuvenation apparently leads to improved T-cell fitness. “We found canonical cytotoxicity functions of human T cells are significantly impaired with age – something not clearly established in the literature before our work,” Kimmel said. However, the team has found many novel combinations that restore T cells’ fitness even stronger than the Yamanaka factors.
The question of durability, according to Kimmel, is crucial – effects that only last while mRNAs are expressed would be difficult to translate into treatments. “But we found that some combinations produce durable changes, measurable many days after turning off the factors,” he said.
Y is this happening?
Nick Chavkin, Assistant Professor at the Department of Pediatrics at Seattle Children’s Research Institute (hence, a local), gave a talk about a particularly interesting mutation affecting blood cells: the loss of the Y chromosome. Due to hematopoietic clonal expansion (when hematopoietic stem cells with certain mutations become more successful in reproduction and, as a result, dominate the cell pool), this mutation is quite prevalent in aged people, but for obvious reasons, only in men. According to the UK Biobank, by age 70, about 45% of men show appreciable Y chromosome loss, which, according to Chavkin, makes it the most common known human post-zygotic mutation.
Large biobank datasets also revealed the link between this long-known condition and all-cause mortality. Men with Y chromosome loss are about twice as likely to die at any given age compared to men without Y loss.
“The first associations were with cancer mortality and Alzheimer’s disease diagnosis – men with Y loss show higher rates of both,” Chavkin said. “We also demonstrated an increased rate of cardiovascular disease. This led us to investigate the mechanistic aspects. While the correlations are interesting, we wanted to know: could Y loss actually promote these disease states, or is it just an age-related phenomenon associated with genomic instability?”
The researchers created a mouse model with 80-90% Y-loss in hematopoietic stem cells. Compared to controls, those mice showed diminished survival, age-related cardiomyopathy, pulmonary and renal fibrosis, and cognitive decline: all the known hallmarks of Y loss.
Y-loss mice also showed exacerbated heart failure conditions. Looking for mechanistic explanations, the researchers discovered that Y-loss macrophages have a preference for fibrotic polarization, unlike some other known clonal mutations that promote inflammation.
Chavkin’s team then looked for the specific Y chromosome genes that drive these effects. “This was relatively straightforward because the Y chromosome is often considered a genetic “wasteland” post-puberty,” Chavkin said. “In mouse macrophages, only four Y chromosome genes are appreciably expressed, all within about a million base pairs: KDM5D, EIF2S3Y, UTY, and DDX3Y.”
Three knockouts had no effect, but UTY knockout recapitulated the full Y-loss phenotype. UTY is an epigenetic modifier that probably has broad regulatory effects.
Further experiments suggested that UTY inhibits pro-fibrotic macrophage polarization by regulating genomic DNA accessibility. UTY knockout probably allows certain transcription factors to bind and promote this polarization, leading to fibrosis.
The team’s current hypothesis is that Y chromosome loss leads to UTY insufficiency in monocytes. This increases chromatin accessibility for pro-fibrotic genes, allowing fibrotic transcription factor activity and ultimately leading to pro-fibrotic polarization and myocardial fibrosis.
“In summary, Y chromosome loss appears to be an age-related somatic mutation contributing to male mortality,” Chavkin said. “Our work suggests UTY plays a key role in this process. This mutation affects multiple hallmarks of aging – these X0 cells show intrinsic genomic instability, epigenetic alterations, effects on chronic inflammation, and altered intercellular communication.”
View the article at lifespan.io









































