Earlier this month, for the third year in a row, the famed Buck Institute for Research on Aging hosted the Longevity Summit. This two-day event was organized by Longevity Global, a community of longevity researchers, investors, and enthusiasts, and its founder Dr. Christin Glorioso. While not the biggest or the longest conference in the field, Longevity Summit has consistently attracted top-tier speakers and audience members. We are happy to bring you a selection of talks from the event. As is our custom, we apologize to the equally worthy speakers whose talks we were unable to include.
Calico wants to compute aging away
Calico was founded more than a decade ago by Alphabet, Google’s parent company. Well-funded and well-positioned to move the longevity field forward, it ignited a lot of hopes that have since faded somewhat due to a perceived lack of tangible results. While we’ve seen some quality research from Calico, such as Synthia Kenyon’s groundbreaking work, the anxiety is still there, and every appearance by a Calico scientist is an event.
Dr. Madeleine Cule talked about using large human cohorts to study changes in aging and how they are related to disease. Her talk hinted at Calico leveraging its Google roots in its fight against aging. “For a computational scientist,” Cule said, “it’s an exciting time with advances in AI and machine learning, plus interesting datasets to ask and hopefully answer questions.”
Cule started by stating the obvious: that the closest model organisms to humans are humans. However, human clinical trials are complicated and costly, and for the purposes of studying aging, also prohibitively long due to our species’ longevity.
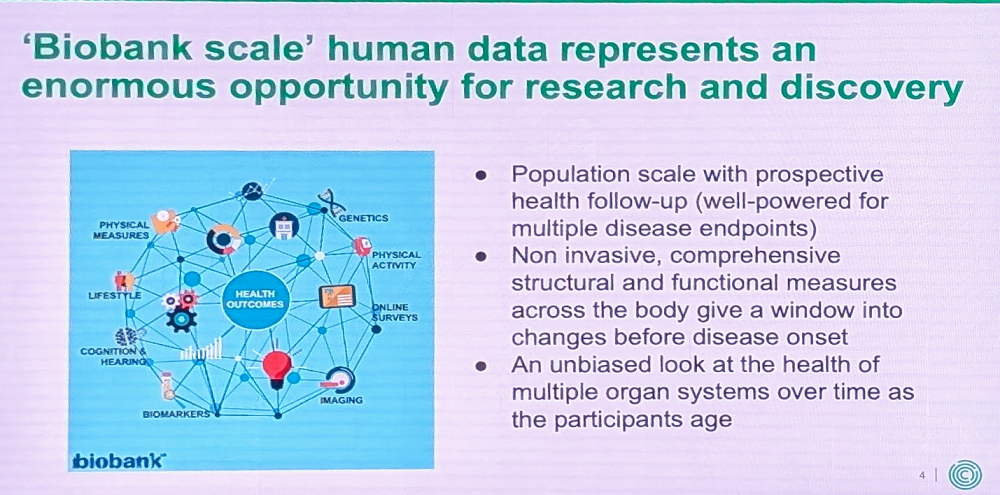
Thankfully, more and more human data is now collected and stored in repositories such as UK Biobank. Analyzing this data does not replace clinical trials, but it can greatly help scientists in understanding aging. UK Biobank, Cule said, is “perfect for an aging company because we can look at changes during aging that affect multiple disease outcomes simultaneously, potentially making new connections.” Biobank data, she added, provides us a window into changes occurring before disease onset and an unbiased, often longitudinal, look at health across the body and its various systems.
Combining this data with the latest machine learning techniques to extract new measurements might help us understand disease outcomes. “In our framework for advancing drug discovery,” Cule explained, “we can use these human measurements to identify new age-related traits with heritable components, then use rapidly accumulating molecular data to interrogate causal relationships. This becomes a platform for developing new therapeutic hypotheses we can test in independent cohorts, experimental medicine approaches, mouse experiments, or in vitro systems.”
Cule then shared a specific example of her team’s work that uses deep learning to extract information from abdominal imaging stored in the UK Biobank database. This dataset contains rich information about body composition and organ health linked to multiple aging-related diseases, such as cardiovascular disease and cancer.
The group started working on it about five years ago by annotating individual organs. Even with Calico’s considerable resources, the process was painstaking and time-consuming, but it eventually paid off. “Thanks to linkage with health outcomes,” Cule said, “we can look across the whole disease spectrum to understand connections.”
She provided an example involving the cardiovascular system. Using body scans, Cule’s team segmented and analyzed blood vessel diameters at different anatomical locations. “This gives us a way to study aneurysm or aging of large blood vessels that isn’t accessible using just clinical information,” she said.
In the future, the team plans to expand beyond using machine learning to replicate what humans would do. “Latest machine learning models don’t need to anchor to what a radiologist might see or think interesting,” Cule noted. “We’re working with machine learning engineers on representation learning approaches that summarize whole images or particular organs into vectors that aren’t necessarily human-interpretable.”
In 2020, Calico teamed up with the UK Biobank to add a longitudinal component by inviting tens of thousands of individuals to repeat the imaging process. “This is exciting for aging research,” Cule said, “because it allows us to study aging-related decline across many imaging phenotypes, identify traits where change predicts disease outcomes, characterize genes involved in the aging process, and identify biomarkers of disease progression – crucial for future clinical trials.”
The hierarchy of clocks
David Fuhrman, Associate Professor at the Buck, gave a talk on creative approaches to building novel biological age clocks. Since we need tools to measure aging in vitro, in animal models, and in humans, this field has been booming, with researchers using epigenetic, transcriptomic, proteomic, lipidomic, and other techniques.
Fuhrman started by showing the audience a slide where different types of biological age clocks were compared side by side. “My favorite is the inflammatory clock,” he then said. “It can give you really good intervention guidance, as opposed to more standard first-generation clocks.”
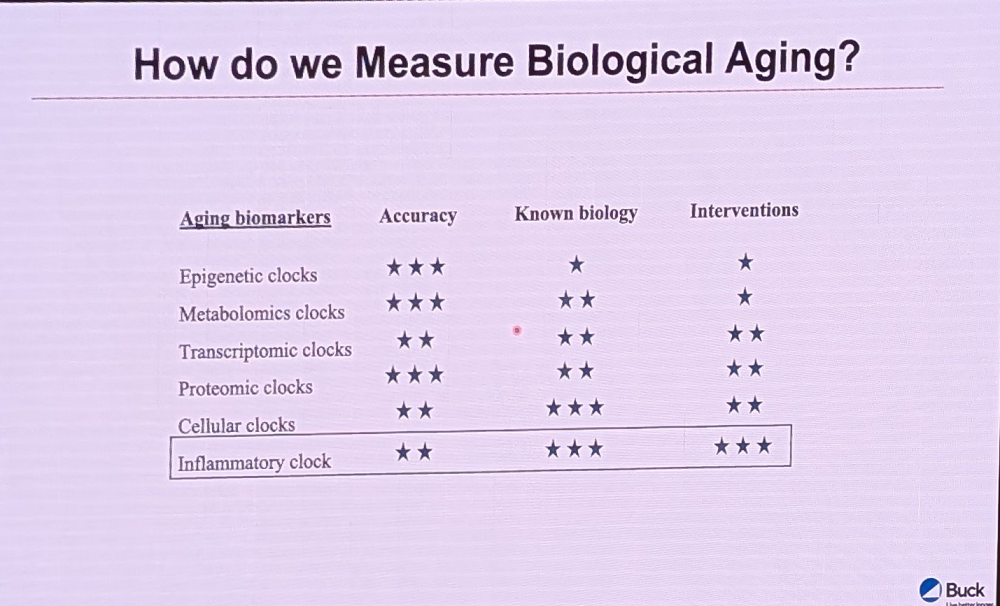
Fuhrman’s complaint about epigenetic clocks is that “we don’t really know how to actuate them very well,” unlike cellular and proteomic clocks. When we understand the proteins involved and the organs we want to target, he explained, it gives clinicians and consumers a much more focused way of intervening.
Fuhrman also heads the Thousand Immunomes project at the School of Medicine at Stanford to identify chronic inflammation biomarkers and build a clock based on them. This clock, he noted, tracks with several clinical endpoints such as multi-morbidity and frailty, even years before they occur, and serves as a surrogate of immune aging and heart health.
“The higher the inflammatory age, the worse the phenotypes overall, both in our Thousand Immunomes cohort and in an independent cohort of exceptional longevity individuals,” Fuhrman said. “We can also predict mortality. We’re now commercializing this through companies, mostly through longevity clinics, wellness centers, and functional medicine practices.”
At his Buck lab, Fuhrman’s team is using baseline multi-omics measurements to predict different functional outcomes with aging. This clock is proxy-methylation-based, that is, it uses methylation data as a proxy for underlying biological processes associated with aging. The clock predicts intrinsic capacity, which according to Fuhrman, is one of the most important measures of aging. Interestingly, few methylation sites (CpGs) selected by this new clock overlap with first and second-generation clocks.
“Using gene sets based on LLMs associated with different hallmarks of aging, we asked if our intrinsic capacity clock relates to hallmarks of aging,” Fuhrman said. “The answer is yes, mostly for chronic inflammation and cellular senescence. We can also associate high intrinsic capacity with overall health, exposome, and lifestyle choices.”
As an example, certain foods drive higher or lower intrinsic capacity as measured by the clock, with all flavonoids, as well as omega-3 fatty acids from animal sources, showing positive correlations. Conversely, omega-6 fatty acids are negatively correlated with intrinsic capacity.
Switching gears, Fuhrman reported on a new study that uses data from UK Biobank and Health and Retirement Study. The idea is to build what Fuhrman calls a third-generation clock: “We’re trying to predict UK ICD-10 chapters – different diseases people die from – using clinical lab measurements first, then creating a metric we can predict using multi-omics: proteomics, metabolomics, DNA methylation patterns, and transcriptomics.”
First-generation clocks were trained to predict chronological age, while most second-generation clocks were trained to predict all-cause mortality. “But if you have a clock that tells you about brain acceleration or heart acceleration, clinicians and consumers can better guide interventions,” Fuhrman noted. “Taking the massive amount of data collected in the UK Biobank and Health and Retirement Study, we’re now creating what I believe is the largest disease-omics mapping that exists today.”
Paying attention to the hypothalamus
Another Buck representative, Dr. Ashley Webb, gave a talk on an area of the brain that is apparently getting too little attention. Cognitive science has long focused on the hippocampus, which makes sense since this part of our brain is crucial for learning. “While our lab studies this area too,” Webb said, “we felt there was another region that has been hugely neglected in the context of aging and neurodegeneration. A number of years back, we started thinking about the hypothalamus, which has numerous functions that are critical for healthy aging.”
Hypothalamus is involved in controlling appetite, hormone production, and circadian rhythms. Some research in animal models shows that manipulating neurons within the hypothalamus affects lifespan. “What makes the hypothalamus very interesting to study, but also very complex, is that this is a highly diverse region,” Webb said. “There are many different subregions and neural subtypes with diverse functions.”
The team’s initial experiment involved single-nucleus RNA sequencing on 3-month-old and 20-month-old mice and produced a high-quality dataset. It showed that some neuronal subtypes remain stable and are more resilient to aging.
The experiment involved only female mice. This limitation actually led to an interesting discovery – the gene most upregulated with age across various subtypes of hypothalamic neurons was a long non-coding RNA known as Xist (pronounced “exist”). “It’s involved in X chromosome inactivation early in life,” Webb explained. “Males are XY, females are XX, and the way we deal with having two X chromosomes in females is through dosage compensation.”
Early during development, Xist is expressed from one of the X chromosomes and silences it throughout life. The researchers were surprised to find that Xist was also the most altered with age. Eventually, they learned to predict a cell’s age from changes in Xist.
Webb told the audience about another interesting finding: while glial cells seemed to age similarly in males and females in terms of gene expression, neurons showed marked differences. “When we looked at neurons – both excitatory and inhibitory – we saw a lack of coordination in how the neurons were changing with age in males versus females,” she said.
Webb concluded her talk by presenting the new machine learning pipeline that her team created to analyze cell-type specific aging in the brain, specifically in the hypothalamus. Transcriptomic clocks are notoriously noisy. Comparing them to methylation clocks, the researchers realized that the latter were binary (a site is either methylated or demethylated), while transcriptomic data is continuous.
“Drawing inspiration from these clocks, we decided to binarize our matrix,” Webb said. “When we did that, it denoised the data. We ran this binary dataset through the same pipeline, and to our surprise, our performance shot up to about 95%. We call this new method Cell-By-Age.”
Cell-By-Age is essentially a transcriptomics-based machine learning method used to predict single-cell age using single-cell RNA-seq datasets. “It’s useful because it can discover cell-type specific aging signatures and evaluate interventions,” Webb explained. “We’ve already tested this by taking a dataset from Andrew Dao’s lab at Stanford, where they exercised mice, and Cell-By-Age could predict rejuvenation of cell types in the brain.”
Reprogramming selected cell types
Vittorio Sebastiano, Stanford professor and CSO of Turn Biotechnologies, is one of the leading authorities on partial cellular reprogramming. Under his guidance, Turn is holding its own in this competitive niche against giants like Altos Labs, producing exciting results.
Sebastiano began with an overview of epigenetics’ role in aging, echoing David Sinclair’s information theory of aging. “Aging,” he said, “is fundamentally an epigenetic problem – something that happens in the epigenome. Because of this deterioration of epigenetic information, we see the manifestation of aging at cellular, tissue, and organismal levels.” Since the epigenome dictates cellular behavior, changes in it result in changes across the whole spectrum of aging markers.
Sebastiano suggested that nature already solved aging on a cellular level via epigenetic reset that occurs early in embryogenesis. Without that, continuation of life would have been impossible. Understanding this epigenetic reset “requires understanding female reproductive biology and gives us tremendous opportunities to develop anti-aging interventions,” he noted.
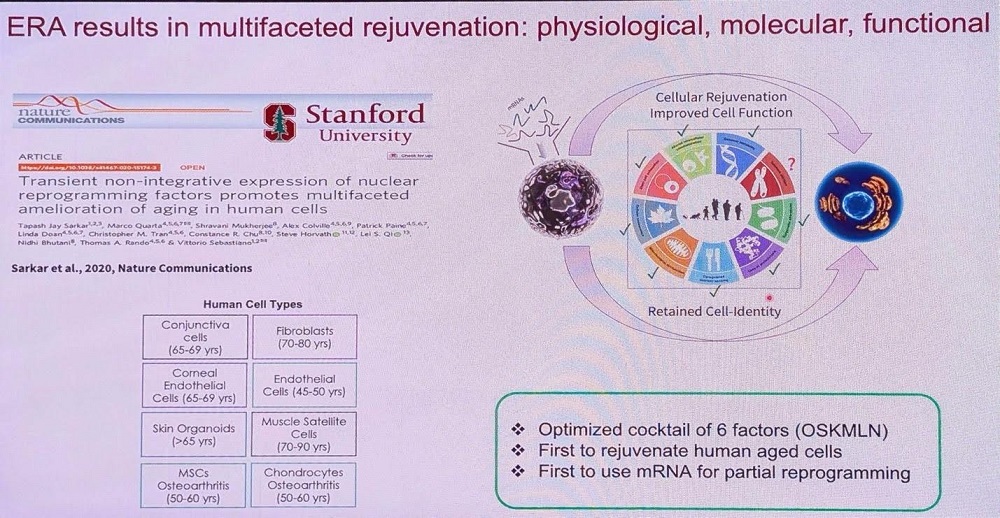
Sebastiano then proceeded to describe Turn’s proprietary platform called Epigenetic Reprogramming of Aging (ERA). “We started this work in late 2014, published in 2020, and eleven years later, I can tell you – it works,” he said.
ERA uses mRNA technology to deliver six reprogramming factors. As of now, ERA’s effectiveness has been demonstrated in 12 different human cell types. The team primarily works with human cells to shorten the way to clinical use. Turn is actively working with several cell types and indications in parallel, and Sebastiano provided two concrete examples.
In collaboration with Marco Quarta and his company Rubedo, Turn rejuvenated aged stem cells from muscle and transplanted them into age-matched hosts. Compared to untreated stem cells, age-matched stem cells treated with ERA restored muscle strength to young muscle levels – about a 40% increase.
Sebastiano is convinced that treating all cells in the body with reprogramming factors is a mistake, although this approach has produced some positive results in animal models. Instead, reprogramming should be targeted to cell types where it would be most beneficial, such as stem cells and immune cells, and tailored for specific indications.
In this spirit, Turn is working on reprogramming aged T cells. “We saw increased expression of two markers associated with T cell functionality – the higher their expression, the more likely the cells can kill tumor cells in the body,” Sebastiano said. “With the ERA treatment, we can restore or enhance the population of stem cell-like cells in the T cell population, which has potential therapeutic implications.” Importantly, the cells also showed a higher proliferative capacity when challenged with cancer cells and were much better at killing those.
The company is now extending this work to blood stem cells. The day of the talk, Turn announced a partnership with the Children’s Hospital of Philadelphia.
Sebastiano’s team was able to compare epigenetic changes caused by the treatment to those caused by other interventions. Interestingly, cellular reprogramming seems to be more aligned in this regard with caloric restriction, while rapamycin alters methylation in different regions.
Third time’s the charm?
A lot of eyes have been on UNITY Biotechnology, a company that aims to bring senolytic treatments to the clinic. In 2020, UNITY’s Phase 2 clinical trial, one of the first in longevity biotech, failed, sending ripples through the entire field. Another one failed in 2023. Undaunted, UNITY’s team has since then continued to hone their approach. The company’s Chief Scientist Mike Sapieha was at the Buck to give an update.
Sapieha talked about the direction stemming from his academic research: vision loss. Some types of vision loss are age-related (most people who lose their sight are above age 50, Sapieha said). Vision loss not only causes depression but also reduces mobility, accelerates dementia, and contributes to other age-related morbidities.
Something that many vision loss disorders have in common is a failure of vascularization. “This starts as a dropout in healthy blood vessels,” Sapieha said, “then growth of blood vessels into areas of the eye that are physiologically avascular, meaning in a healthy eye, blood vessels typically don’t grow there.”
Around 2004-2006, treatment of many of those diseases was revolutionized with the first anti-vascular endothelial growth factor (VEGF) treatments. Today, it is a lucrative market of around 14 billion dollars.
The problem, according to Sapieha, is that those drugs target all vasculature growth. UNITY, on the other hand, wants to selectively target only “the bad blood vessels.”
“The anti-VEGFs hit all blood vessels,” Sapieha said, “so we asked: is there a way to molecularly identify these abnormal structures and develop a tool to selectively eliminate them? Much like gardening, where you cut out dead branches and the healthy ones regenerate.”
Sapieha’s team found that pathological angiogenesis causes retinal neurons to go into a hibernation-like state, triggering endoplasmic reticulum stress pathways. If this ER stress is not resolved, cells in the retina enter senescence.
After those senescent cells start producing inflammatory factors, neutrophils arrive at the scene to mark the senescent cells for destruction. However, with age, this immune-mediated elimination of senescent cells becomes compromised. “We spent years identifying susceptibility nodes in senescent blood vessel cells and landed on a protein called BCL-XL, which lives inside mitochondria and sequesters death effectors,” Sapieha said.
The scientists decided to go specifically after senescent endothelial cells. After proving that the drug was safe, the team focused on diabetic macular edema, a complication of diabetic retinopathy, which affects around 1.7 million people in the U.S.
“We found senescent cells were highly enriched in areas of disease activity, right next to the leaking blood vessels,” Sapieha said. “Single-cell RNA sequencing in mouse models showed about 5-10% of endothelial cells expressing these senescence markers.”
One of the main roles of endothelial cells is to maintain the blood barrier via proteins called adherens junctions. The researchers discovered that in senescent cells, these proteins do not form properly, compromising the barrier function. “In efficacy models, we saw inflammatory factors like IL-6, IL-1β, and TNF-α increased, then significantly reduced with UBX treatment,” Sapieha said.
UNITY’s Phase 2 clinical trial enrolled patients who had received anti-VEGF standard of care for about six months and plateaued in improvement, despite continuing to receive injections every six weeks. Sapieha’s team hypothesized that, with their senolytic approach, one injection would be enough to eliminate senescent cells and allow healthy neighboring cells to regenerate.
A year after the single injection, 53% of patients improved to the point where they needed no other treatments. “Most striking, patients with BCVA Best Corrected Visual Acuity, a measurement of how well a person can see with corrective lenses) under 60 gained an average of 10 letters after one year with that single injection – potentially enough improvement to restore driving ability,” Sapieha said. More results are expected at the end of Q1, 2025.

Measuring brain aging
The debate around amyloid’s role in Alzheimer’s disease has dominated the field for decades. However, according to Dr. Christin Glorioso, who, this time, was wearing her NeuroAge Therapeutics founder hat, we might need to think about it differently. “Amyloid is a risk factor, like cholesterol,” she said. “We should think about it not as the single cause of Alzheimer’s disease, but one cause among many.”
Alzheimer’s is multifactorial and doesn’t look the same in all people, Glorioso noted. This means that we need better biomarkers and segmentation to predict who anti-Alzheimer’s drugs will work for. It is also important to diagnose the disease and identify responders and non-responders earlier, since current drugs have been shown to work better at an early stage.
Glorioso’s company is focusing on aging itself as a key risk factor. Her research shows that pathological processes in brain cells start surprisingly early. “Even in your 20s,” she explained, “you have calcium dysregulation, mitochondrial dysfunction, DNA damage, changes in neurotransmitters, and brain-specific hallmarks like loss of synapses between neurons and inflammatory processes in glia.”
By studying donated brain tissue, Glorioso’s team discovered many genes that show pro-Alzheimer’s age-related changes, such as calbindin and GFAP. This allowed the researchers to create an algorithm that predicts brain age from 52 blood RNA transcripts. “This is an organ-specific aging clock, similar to Tony Wyss-Corey’s work with proteomics, but using RNA-seq as a more accessible approach,” Glorioso said. “You go to your doctor yearly to check cholesterol for heart disease risk and glucose for diabetes risk, but no one checks brain aging – we didn’t have a way to assess neurodegeneration risk.”
The final product is an “AI-based super predictor” that combines blood biomarkers, genetics, brain MRI, and cognitive testing to predict brain age. This information is actionable, Glorioso said, “because about 40% of your brain aging rate is due to lifestyle, with 60% genetic. There’s this huge modifiable component you can work on right now to reduce your brain aging.”
Glorioso cited recent research that supports this approach. A new study showed that just 25 extra minutes of exercise per week increased brain volume on MRI – “literally growing their brains back,” as Glorioso put it. In another case, an entrepreneur with high genetic risk for Alzheimer’s managed to reverse his amyloid accumulation through intensive lifestyle changes.
Glorioso’s company provides its clients with a comprehensive dashboard that includes measurements of their brain age and lifestyle recommendations. The “big six” are exercise, healthy eating, getting eight hours of sleep, community engagement, stress reduction, and staying mentally active. “There’s also good evidence for Omega-3s from fish, walnuts, and flaxseed,” Glorioso said. “Coffee and tea are very good for brain aging due to their polyphenols and other antioxidants, not the caffeine itself. We curate many other studies and provide information through both our dashboard and newsletter.”
The company is also moving beyond diagnostics. Using their multi-omic dataset, they are developing therapeutics targeting the pathways that drive brain aging. This includes a small molecule and an antibody for Alzheimer’s and mild cognitive impairment.

Make splicing great again
Lorna Harries, a professor of molecular genetics at the University of Exeter Medical School and founder of Senisca, focused her talk on splicing – the process of creating different mRNAs from a single pre-mRNA transcript by removing non-coding sequences (introns) and joining coding sequences (exons). “98 percent of your transcriptome is alternatively spliced,” Harries said, “so most genes can make a number of isoforms that are expressed at different times, in different places, in response to different stimuli. This is a fundamental underpinning of our molecular response to stress.”
Splicing, which is regulated by about 150 splicing factors, becomes dysregulated with age. Understanding those proteins makes it possible to restore the splicing ability, which Harries likened to partially reprogramming the cell. She called splicing dysregulation “a new and druggable hallmark of aging.”
However, to declare something a hallmark of aging, she said, you first have to show that it happens during normal aging in populations – which Senisca did in collaboration with Luigi Ferruci at the NIA. “Six of the seven most age-affected pathways were directly related to RNA processing, particularly splicing,” Harries said. The team validated these findings in multiple populations.
Working with human cells, the researchers found that replicative senescence is accompanied by downregulation of splicing-related genes. Vice versa, disrupting even a single splicing factor could trigger cellular senescence. More importantly, in the longitudinal cohort InCHIANTI, people with lower splicing factor expression showed faster decline in cognitive and physical function over time.
“Splicing factors are actually pretty good candidate aging genes,” Harries said. “They regulate splicing patterns globally, but they also do many other things – regulate telomeres, SASP (senescence-associated secretory phenotype) proteins and molecular stress resilience, play a role in DNA repair, and are essential for RNA quality control.”
“The million-dollar question was: what happens if we switch them back on?” she continued. When her team restored splicing factor levels in old cells, they saw a dramatic “reprogramming effect”: a 60 percent reduction in senescent cells. The formerly senescent cells re-entered the cell cycle, and their telomere length was restored to near-youthful levels.
This led to the development of two therapeutic approaches. The first uses small molecules for skin health applications, developed through a partnership with a major skincare company. The second, more ambitious approach uses oligonucleotides, small pieces of DNA or RNA that can precisely target specific genes.
Senisca is initially testing its oligonucleotide therapy on idiopathic pulmonary fibrosis (IPF), a devastating age-related lung disease where cellular senescence plays a major role. In laboratory tests using lung tissue from IPF patients, its treatment reduced senescent cells by 75-80 percent and decreased markers of tissue scarring. “We see drops in MMP7, a marker of pathological lung remodeling that we can track from laboratory studies all the way to the clinic,” Harries noted.
However, splicing factors are finicky, as they are highly auto-regulated and cross-regulated. Rather than completely blocking or activating these genes, Senisca’s treatment pushes them back toward their normal working range, restoring the equilibrium. “These genes exist in a Goldilocks zone – you don’t want too much or too little,” Harries said. “We just give them a little nudge, and the cell does the work for us.”
View the article at lifespan.io








































