In JCI Insight, researchers have explored the possibility of using gene therapy to restore a crucial protein and repair hearing loss.
Hearing and its failure
In mammals, afferent neurons, which originate from the inner ear, transform received stimuli (sound waves) into electrical signals [1]. This process is known as mechanoelectrical transduction, and a specific myosin, MYO7A, has been found to be a critical part of it [2]. Problems with the related gene, Myo7a, result in various forms of deafness [3].
Knocking this gene out in mature animals has been found to lead to reversion into a nonfunctional state, which is normally found in prenatal animals that have not yet developed the ability to hear [4]. This state is characterized by efferent neurons, which originate from the brain stem rather than inner ear cells, having direct connections to the inner ear that do not exist in functionally hearing animals. As this process also occurs with aging [5], the researchers decided to investigate whether or not directly affecting this gene could lead to hearing restoration in an animal model.
Mice that lose their hearing
The researchers developed a mouse strain that can be triggered to downregulate the Myo7a gene. A few days after this gene was downregulated, mice of both sexes quickly lost their hearing, and by two weeks, the mice were nearly completely deaf at all frequencies, and a large part of their hearing was found to be rewired into the same nonfunctional state found in prehearing and aged animals. Myo7a was not found to affect the afferent neurons themselves, only the efferent innervation of hair cells.
Downregulating Myo7a only in the inner hair cells, which directly send signals to the brain, was found to be sufficient to lead to deafness. Downregulating it in the outer hair cells, amplifier cells that are more susceptible to damage and aging, led to deafness as well.
Restoration through gene therapy has positive effects
Injecting the inner ears of these genetically modified mice with an adeno-associated virus (AAV) that restores this protein had positive effects, with many wiring structures being restored to their functional adult versions. However, it did not fully restore the mice’s hearing to the level of an unaffected control group. Most of the treated animals could hear very loud noises as measured by the auditory brainstem response thresholds for clicker tests (left) and specific pure tone frequencies (right).
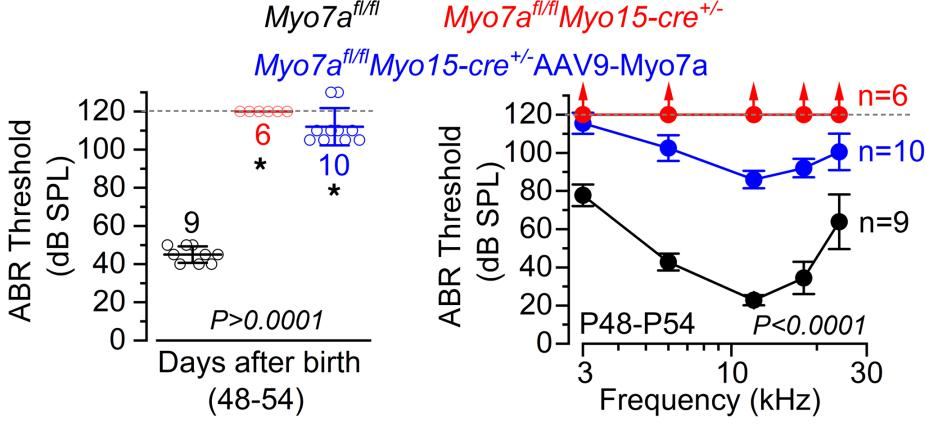
Of course, this is a study on a genetically modified mouse model, not aged wild-type animals nor human beings. Further research will need to be done to determine if aged animals can be affected by this kind of gene therapy and whether or not the results can be replicated in people. Furthermore, the effects of this particular AAV approach were weak, but if it can be applied to people, it may be enough to allow hearing aids to function more effectively.
Additional findings
The researchers discovered that MYO7A controls many aspects of hearing loss that were previously hypothesized to occur due to other factors, such as secondary effects from other systems failing or a failure in proper molecular transport. This research also sheds some light on a link between loud noise and deafness: to protect the ears, the brain uses the efferent system to temporarily reduce hearing capability in loud situations [6], and this may be related to the efferent innervation of the inner ear over time.
The most critical finding is that the adult cochlea, which processes hearing, is in fact capable of being remodeled through changes in gene expression after birth. True biological hearing restoration, once merely a dream, appears to finally be on the table with this and other recent gene therapy approaches [7], and people with congenital deafness caused by some Myo7a mutations, or people who have lost their hearing through repeated noise exposure, may have an effective treatment in the future.
Literature
[1] Fettiplace, R. (2011). Hair cell transduction, tuning, and synaptic transmission in the mammalian cochlea. Comprehensive Physiology, 7(4), 1197-1227.
[2] Hasson, T., Gillespie, P. G., Garcia, J. A., MacDonald, R. B., Zhao, Y. D., Yee, A. G., … & Corey, D. P. (1997). Unconventional myosins in inner-ear sensory epithelia. The Journal of cell biology, 137(6), 1287-1307.
[3] Weil, D., Küssel, P., Blanchard, S., Lévy, G., Levi-Acobas, F., Drira, M., … & Petit, C. (1997). The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nature genetics, 16(2), 191-193.
[4] Corns, L. F., Johnson, S. L., Roberts, T., Ranatunga, K. M., Hendry, A., Ceriani, F., … & Marcotti, W. (2018). Mechanotransduction is required for establishing and maintaining mature inner hair cells and regulating efferent innervation. Nature Communications, 9(1), 4015.
[5] Lauer, A. M., Fuchs, P. A., Ryugo, D. K., & Francis, H. W. (2012). Efferent synapses return to inner hair cells in the aging cochlea. Neurobiology of aging, 33(12), 2892-2902.
[6] Fuchs, P. A., & Lauer, A. M. (2019). Efferent inhibition of the cochlea. Cold Spring Harbor perspectives in medicine, 9(5), a033530.
[7] Amariutei, A. E., Jeng, J. Y., Safieddine, S., & Marcotti, W. (2023). Recent advances and future challenges in gene therapy for hearing loss. Royal Society Open Science, 10(6), 230644.
View the article at lifespan.io








































