In the Journal of Nanobiotechnology, researchers have described a new method of delivering a long-lasting treatment into cartilage.
A protein that promotes autophagy
Previous work has linked expression of the FGF18 protein with healthy cartilage and joints [1]. Problems with the gene responsible for FGF18 lead to osteoarthritis [2], and it has been found to be important alongside arthritis therapies, such as hydrogels that form a lattice for cartilage growth [3]. This is because FGF18 is known to have positive effects on the FOXO3 pathway, which stimulates autophagy, the cellular self-consumption process that removes unwanted and harmful components [4].
However, using a protein as a cartilage treatment has its own problems. Recombinant proteins directly delivered into tissue don’t last very long [5], and even mRNA-based therapies are vulnerable to rapid degradation in the human body [6]. To combat this, the researchers have chosen lipid nanoparticles (LNPs), which encapsulate the mRNA in order to deliver it into cells [7].
The researchers first confirmed the existence of a link between FGF18 and osteoarthritis. A broad gene expression database has reported that elderly people have only a quarter the FGF18 of young people. Tissue samples have revealed that people who undergo total knee arthroplasty have their FGF18 reduced by half. Similarly, mice that have had arthritis artificially induced by meniscus destabilization, as well as naturally aged mice, have approximately half the FGF-18-positive cells of healthy young mice.
Exposing cartilage-generating cells (chondrocytes) to an inflammatory environment characterized by TNF-α resulted in FGF18 expression being reduced to a fourth of its normal value. Driving chondrocytes senescent by exposing them to hydrogen peroxide reduced FGF18 to two-fifths of its normal value.
Effective delivery and therapeutic effects
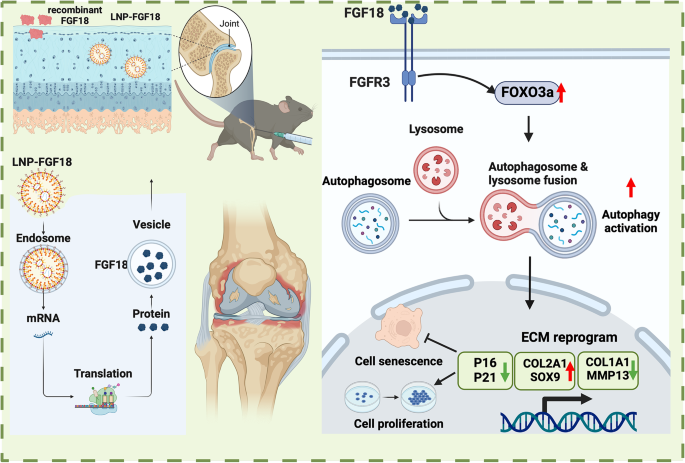
The mRNA delivery appeared to be effective. A cellular examination showed no toxicity to chondrocytes even at high concentrations. Unlike recombinant FGF18, the LNP-encapsulated mRNA nanoparticles penetrated relatively deeply into the cartilage of both young and old mice. The nanoparticles were just small enough to fit within the pores of the mice’s collagen networks, even the dense, cross-linked collagen of older animals.
Using a bioluminescent reporter, the researchers found out that this LNP treatment stays confined to where it needs to be and does not migrate to other organs, such as the liver, in appreciable amounts. Instead, it stays within the knee joint for approximately six days, and its effects diminish far slower than mRNA without LNP encapsulation. The LNP-mRNA was found to successfully cause cells to express significant amounts of the FGF18 protein.
This approach had significant beneficial effects in a cellular culture. Cellular senescence induced by the inflammatory cytokine IL-1β was cut approximately in half, as measured by p16, p21, p53, and SA-β-gal staining. Proliferation was approximately doubled as well. LNP-mRNA for FGF18 had very similar effects to pure FGF18 in this cellular experiment.
Autophagy was similarly upregulated. FOXO3 is downregulated when chondrocytes are exposed to IL-1β, but the LNP-mRNA was found to restore it nearly to the level of the control group. Cells that were only exposed to LNP-mRNA without IL-1β had even higher levels of FOXO3. This led to an increase of cartilage-producing proteins, and further experiments confirmed that this was due to an increase in autophagy.
After confirming its effects in cells, the researchers turned to mice: a control group, a group with a destabilized meniscus and no treatment, a group treated with FGF18 protein every week, and a group treated with LNP-mRNA every week. The damaged, untreated group was hypersensitive to pain, which was partially ameliorated by FGF18 and slightly moreso by the LNP treatment, although all of the damaged mice gradually got more sensitive to pain over eight weeks.
The LNP treatment was also found to benefit the mice’s gait and physical biomarkers, in both the destabilized meniscus model and in naturally aged mice. In many of the tests, there were no significant differences between FGF18-treated and LNP-treated mice, but there were some benefits to the new approach.
Most notably, the cartilage of the LNP-treated mice was significantly thicker, restoring the cartilage of damaged mice nearly to that of undamaged mice and, most critically, restoring the cartilage of aged mice nearly to that of young mice. A closer investigation found that the LNP injection was having the same effects in the mice as in the cellular culture, restoring proliferative capacity to the mice’s chondrocytes.
This is not a human study, but it appears that human trials are the next logical step for this approach, as it appears to be both safe and effective in animal models. Time will tell whether this particular LNP approach will be tested for the clinic or if it will undergo further refinement first.
Literature
[1] Davidson, D., Blanc, A., Filion, D., Wang, H., Plut, P., Pfeffer, G., … & Henderson, J. E. (2005). Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. Journal of Biological Chemistry, 280(21), 20509-20515.
[2] Boer, C. G., Hatzikotoulas, K., Southam, L., Stefánsdóttir, L., Zhang, Y., de Almeida, R. C., … & Wilkinson, J. M. (2021). Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell, 184(18), 4784-4818.
[3] Gothard, D., Rotherham, M., Smith, E. L., Kanczler, J. M., Henstock, J., Wells, J. A., … & Oreffo, R. O. (2024). In vivo analysis of hybrid hydrogels containing dual growth factor combinations, and skeletal stem cells under mechanical stimulation for bone repair. Mechanobiology in Medicine, 2(4), 100096.
[4] Cinque, L., Forrester, A., Bartolomeo, R., Svelto, M., Venditti, R., Montefusco, S., … & Settembre, C. (2015). FGF signalling regulates bone growth through autophagy. Nature, 528(7581), 272-275.
[5] Evans, C. H., Kraus, V. B., & Setton, L. A. (2014). Progress in intra-articular therapy. Nature Reviews Rheumatology, 10(1), 11-22.
[6] Hajj, K. A., & Whitehead, K. A. (2017). Tools for translation: non-viral materials for therapeutic mRNA delivery. Nature Reviews Materials, 2(10), 1-17.
[7] Hou, X., Zaks, T., Langer, R., & Dong, Y. (2021). Lipid nanoparticles for mRNA delivery. Nature Reviews Materials, 6(12), 1078-1094.
View the article at lifespan.io








































