In Cell Reports Medicine, researchers have described how they created a fully functional pancreas made from human cells and found it to work in mice.
A new era of organ replacement
In their introduction, the researchers discuss the well-known problems with insulin injections to treat Type 1 diabetes: the sort of constant monitoring that is required is difficult for patients to consistently comply with [1], and daily manual injections can’t adequately simulate the responsiveness of pancreatic tissue [2]. Direct injection of beta islet cells, which produce insulin, are limited by donor organs and require the immune system to be suppressed [3].
More modern techniques recognize that the extracellular matrix (ECM) governs a large part of how stem cells differentiate [4], and the effects of the ECM on the pancreas have been investigated in detail [5]. This led to a rapid increase in the work being done in this area, with ECM structures being created for the purpose [6]. The researchers of this paper had created a functional pancreas with insulin-producing cells from pigs, and it was functional in mice [7].
Therefore, the next step was to use cells derived from human beings.
Constructed organs are more effective than previous approaches
The researchers used two separate kinds of cells derived from human induced pluripotent stem cells (iPSCs): insulin-producing islet cells (SC-islets) and endothelial cells (iECs), which line the walls of arteries and veins. First, the researchers brought together these cells in a 9-to-1 ratio in order to produce spheroids (ViβeSs). Then, they populated decellularized rat lung tissue with more iECs and let them grow for two days, and finally, they injected this ready tissue with ViβeSs and more iECs to promote blood vessel formation (vascularization), creating a vascularized endothelia pancreas built from iPSCs: an iVEP.
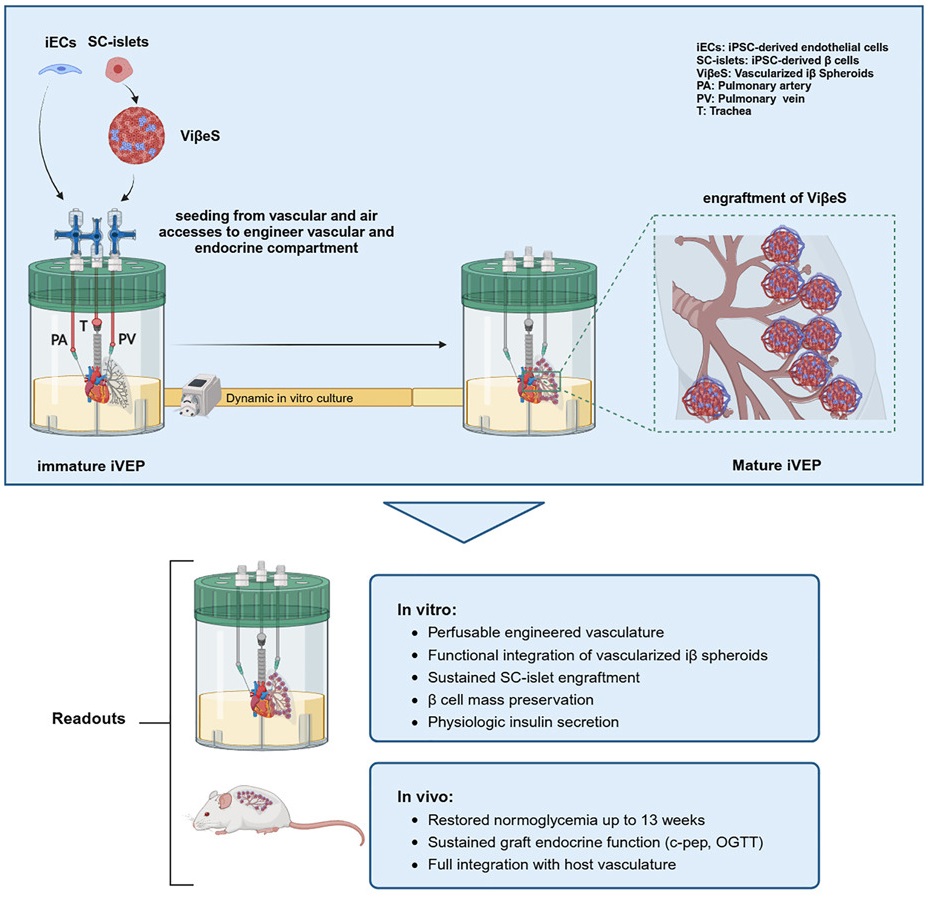
This approach worked. The injected ViβeSs did not come loose from the structure; instead, the iECs formed vascular tissue within the decellularized lungs, providing them with stability and the blood flow that they need to perform their duties. The iVEP structure was found to grant significant improvements to the cells’ survival and responsiveness, with the cells producing more insulin under high-glucose conditions.
In immunocompromised diabetic mice, the iVEP structure also performed much better than ViβeSs put into a a pre-vascularized pouch under the skin. In only two out of the thirteen mice given the latter, normal glycemia was established within a month; this happened in all the mice given iVEPs. Half of the iVEP-receiving mice had normal glycemia within two weeks of transplantation.
Unsurprisingly, removing the iVEPs from these mice led to diabetes within a week. An exaination of these structures revealed extensive vascular connections: the mice had successfully integrated the iVEPs into their bodies. Further investigation found that iECs were necessary in the creation of iVEPs; without endothelial cells, the structures fail to properly integrate into the vascular structure.
Decellularized, vascularized structures as a way forward
With these results in hand, the researchers compared their iVEP approach to previous work. They hold that their approach to vascularization improves many fundamental aspects of cellular development: for example, they note that by themselves, islet cells generated from iPSCs require 20 days to reach a mature and effective phenotype [8]. Meanwhile, in iVEPs, the cells require only a week to reach this phenotype.
While the researchers note that their approach is more complicated than products that are already in clinical trials, they believe that it will make for a better product. However, their existing scaffolds, derived from rats, are far too small to use in people. They plan to use pig organs instead, and they hope to use hypo-allergenic cells that completely get rid of the risk of immune rejection and the need for potentially risky immunosuppressants.
The researchers developed this approach to treat type 1 diabetes, but this work has implications for many other diseases, many of which are age-related. While replacing the pancreas cannot heal the insulin resistance inherent in type 2 diabetes, it may be a viable strategy for long-term loss of function. Similarly, this technology can potentially be applied to many other organs, including the lungs and heart. While wholesale replacement of human organs with bioengineered equivalents is still not on the table, this technology continues to advance to the clinic.
Literature
[1] Beck, R. W., Bergenstal, R. M., Laffel, L. M., & Pickup, J. C. (2019). Advances in technology for management of type 1 diabetes. The Lancet, 394(10205), 1265-1273.
[2] Piemonti, L. (2021). Felix dies natalis, insulin… ceterum autem censeo “beta is better”. Acta Diabetologica, 58(10), 1287-1306.
[3] Pepper, A. R., Bruni, A., & Shapiro, A. J. (2018). Clinical islet transplantation: is the future finally now?. Current opinion in organ transplantation, 23(4), 428-439.
[4] Hogrebe, N. J., Augsornworawat, P., Maxwell, K. G., Velazco-Cruz, L., & Millman, J. R. (2020). Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nature biotechnology, 38(4), 460-470.
[5] Berger, C., Bjørlykke, Y., Hahn, L., Mühlemann, M., Kress, S., Walles, H., … & Zdzieblo, D. (2020). Matrix decoded–A pancreatic extracellular matrix with organ specific cues guiding human iPSC differentiation. Biomaterials, 244, 119766.
[6] Peloso, A., Urbani, L., Cravedi, P., Katari, R., Maghsoudlou, P., Fallas, M. E. A., … & Orlando, G. (2016). The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Annals of surgery, 264(1), 169-179.
[7] Citro, A., Neroni, A., Pignatelli, C., Campo, F., Policardi, M., Monieri, M., … & Piemonti, L. (2023). Directed self-assembly of a xenogeneic vascularized endocrine pancreas for type 1 diabetes. Nature Communications, 14(1), 878.
[8] Velazco-Cruz, L., Song, J., Maxwell, K. G., Goedegebuure, M. M., Augsornworawat, P., Hogrebe, N. J., & Millman, J. R. (2019). Acquisition of dynamic function in human stem cell-derived β cells. Stem cell reports, 12(2), 351-365.










































