In the Nature publication Signal Transduction and Targeted Therapy, researchers have described how glibenclamide, a drug used to treat type 2 diabetes, partially reverses epigenetic alterations and fights cellular senescence in mice.
A system tightly tied together
This paper begins with a discussion of the relationship between epigenetic alterations and cellular senescence. The histone H3K4me3 upregulates the senescence-related genes Cdkn1a, which is responsible for the biomarker p21 [1], and Cdkn2a, which is responsible for the biomarker p16 [2]. H3K27me3, on the other hand, downregulates these genes. Another histone, H3K9me3, suppresses repetitive genetic elements that cause an inflammatory response related to senescence [3].
While research has been done on directly targeting these histones [4], doing this with small molecules is difficult because they are structurally similar [3]. These researchers point to evidence suggesting that it may be more effective to target metabolism instead, as fundamental aspects of metabolism are linked to histone methylation [5].
These researchers had previously found that chlorpropamide provides a rejuvenation effect in C. elegans worms through a mitochondrial pathway [6]. They began this work to more definitively determine how and why this was happening, looking for a therapeutic target.
A metabolic target
This work began with a study on lung fibroblasts. Using a chemical probe based on chlorpropamide, the researchers looked at protein functions in order to determine what was being affected by this compound. They found MDH2 to be a potential target, as it had similar mitochondrial effects as chlorpropamide.
Further work in lung fibroblasts found that MDH2 was directly related to cellular senescence, whether it was induced by doxycycline or by excessive replication. The researchers created two cell lines, one with suppressed MDH2 and the other with overexpressed MDH2, to determine whether this relationship was causative in nature. They found that suppressing MDH2 reduced key senescence biomarkers, including SA-β-gal and p16, while overexpressing it increased them. The difference was not large, but it was statistically significant.
The researchers then tested how MDH2 interacts with five different sulfonylureas, a class of drugs that includes chlorpropamide. Of these drugs, the researchers found that glibenclamide has the strongest interaction with MDH2, far stronger than that of chlorpropamide.
In doxycycline-induced senescent lung fibroblasts, glibenclamide was found to reduce senescence biomarkers, including SA-β-gal, p16, and interleukins. Its overall effects in this area seemed to be roughly on par with those of metformin, another diabetes drug; it was not as good at reducing the inflammatory cytokine IL-6, but it reduced IL-1β in these cells, which metformin did not do. These beneficial effects were confirmed to be dependent on MDH2, as glibenclamide had no effects in cells with silenced MDH2.
Increases in histones and ROS
While the effects on lifespan and frailty were positive, glibenclamide increased, rather than decreased, mitochondrial reactive oxygen species (ROS) in lung fibroblasts. The researchers found that this was due to the inhibition of the TCA metabolic cycle, which forced the mitochondria to use oxygen-burning glycolysis instead [7]. The researchers hold that these effects on the TCA cycle are core to its beneficial effects, as they relate to the methionine cycle, which affects methylation.
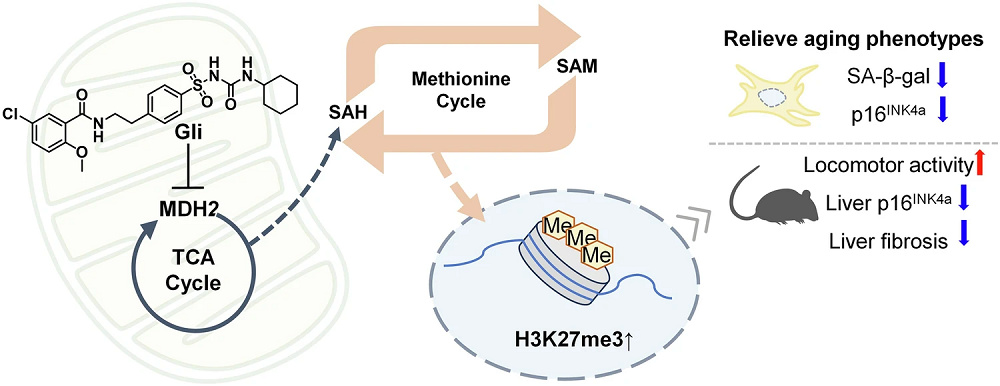
On the first day of treatment in lung fibroblasts, glibenclamide immediately upregulated the senescence suppressor H3K27me3. Interestingly, when given for five days, glibenclamide upregulated both H3K4me3 and H3K27me3 in these cells while having no effect on H3K9me3.
Benefits for mice
The researchers also experimented on Black 6 mice. One group of 12-month-old mice was given glibenclamide, another was given NMN, and a third served as a control group. At 26 and 27 months of age, the glibenclamide group had significantly less frailty than either of the other groups. Mice given NMN appeared to live slightly longer on average, but the effect was not statistically significant; mice given glibenclamide, on the other hand, lived significantly longer.

Another experiment found that, while its physical benefits were not apparent at this age, glibenclamide reduces liver fibrosis and senescence in 20.5-month-old mice. In these animals, the drug was found to have significant effects on H3K27me3 but not H3K4me3.
While its anti-aging effects have not been tested in human beings, glibenclamide is a drug that is already being prescribed in the clinic. If these beneficial effects can be confirmed in human beings, this drug might be more widely prescribed to slow cellular senescence, particularly in the liver. The researchers also suggest that derivatives of this drug could be developed to more precisely target MDH2 to further slow cellular senescence.
Literature
[1] Yan, K., Ji, Q., Zhao, D., Li, M., Sun, X., Wang, Z., … & Liu, G. H. (2023). SGF29 nuclear condensates reinforce cellular aging. Cell Discovery, 9(1), 110.
[2] Kotake, Y., Zeng, Y., & Xiong, Y. (2009). DDB1-CUL4 and MLL1 mediate oncogene-induced p16 INK4a activation. Cancer research, 69(5), 1809-1814.
[3] Zhang, B., Long, Q., Wu, S., Xu, Q., Song, S., Han, L., … & Sun, Y. (2021). KDM4 orchestrates epigenomic remodeling of senescent cells and potentiates the senescence-associated secretory phenotype. Nature aging, 1(5), 454-472.
[4] Hsu, C. L., Lo, Y. C., & Kao, C. F. (2021). H3K4 methylation in aging and metabolism. Epigenomes, 5(2), 14.
[5] Salminen, A., Kauppinen, A., Hiltunen, M., & Kaarniranta, K. (2014). Krebs cycle intermediates regulate DNA and histone methylation: epigenetic impact on the aging process. Ageing research reviews, 16, 45-65.
[6] Mao, Z., Liu, W., Huang, Y., Sun, T., Bao, K., Feng, J., … & Li, J. (2022). Anti-aging effects of chlorpropamide depend on mitochondrial complex-II and the production of mitochondrial reactive oxygen species. Acta Pharmaceutica Sinica B, 12(2), 665-677.
[7] Li, X., Yang, Y., Zhang, B., Lin, X., Fu, X., An, Y., … & Yu, T. (2022). Lactate metabolism in human health and disease. Signal transduction and targeted therapy, 7(1), 305.








































