A new study defines the concept of immune resilience and positions it as a central determinant of aging trajectories, linking it to survival, inflammation control, and the body’s ability to withstand stress [1].
Keeping the balance
Geroscientists have long suspected that the immune system plays an outsized role in aging, one that has deep evolutionary roots and stems from the fundamentally double-edged nature of inflammation. On one hand, inflammation is a crucial part of the immune response that helps stave off the incessant, lifelong onslaught of pathogens. On the other, it destroys cells and tissues. Health and lifespan may depend, in large part, on how well the body performs this balancing act [2]. A well-honed immune system combines effectiveness with relatively low inflammation levels.
A new study by researchers from the University of Texas published in Aging Cell proposes immune resilience (IR) as a major determinant of aging trajectories, linking it to survival, inflammation control, and the body’s ability to withstand stress. By analyzing about 17,500 participants across multiple cohorts, the researchers identified IR as a dynamic trait that can predict health outcomes more strongly than age alone.
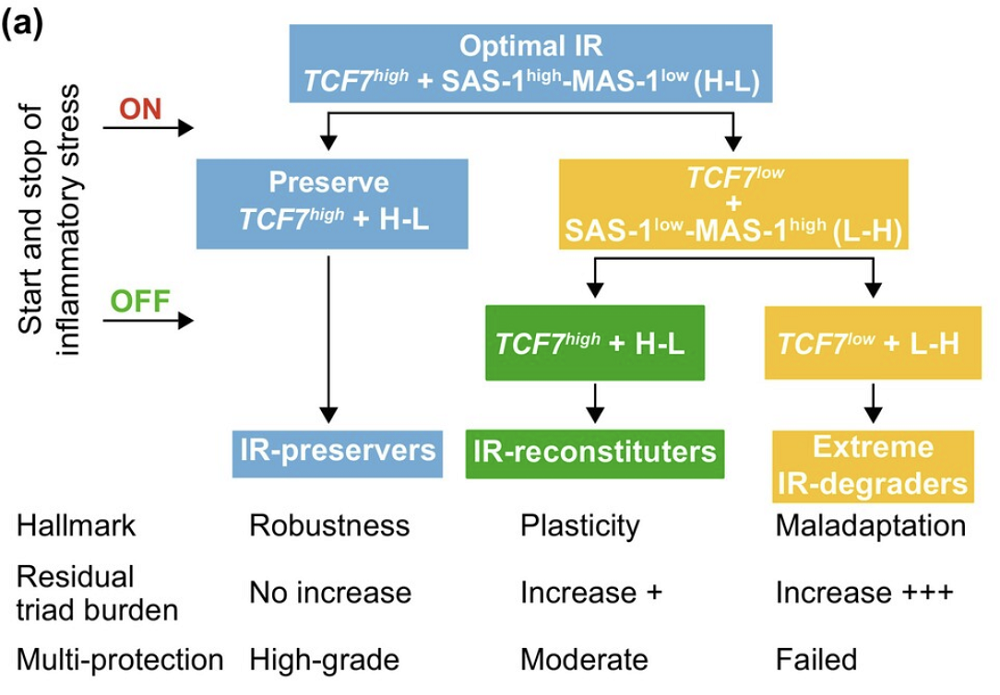
They began by stratifying participants using several standard immune markers, including CD4/CD8 T cell ratios, and longitudinally analyzed how people with different immune profiles react to inflammatory stress events, such as infections or hospitalization. This allowed the team to classify individuals into IR-preservers, reconstituters, or degraders.
IR-preservers maintained robust immune defenses and relatively low inflammation throughout the stress event. IR-reconstituters experienced temporary loss of IR but eventually regained it. In IR-degraders, stress events caused an irreversible exacerbation of the “pathogenic triad”, a cluster of processes accelerating biological aging: chronic inflammation (inflammaging), immunosenescence, and the accumulation of senescent cells.
The team then delved deeper into the molecular traits associated with the three subsets. Using transcriptomics and proteomics, the researchers derived two molecular signatures linked to IR status: survival-associated (SAS-1) and mortality-associated (MAS-1). The former was characterized by upregulation of proteins supporting immune competence, and the latter by proteins associated with inflammation and programmed cell death. Interestingly, components of the insulin-like growth factor 1 (IGF-1) pathway were positively associated with SAS-1 and negatively with MAS-1, aligning IR with established aging regulators.
The master regulator
One gene stood out: TCF7, a transcription factor essential for maintaining stem-like, multipotent T cells. TCF7 was strongly associated with the SAS-1 signature and predicted long-term survival across multiple contexts, including chronic conditions like HIV, tuberculosis, and lupus. People with high TCF7 expression were far more likely to preserve immune function under stress.
Interestingly, in the Framingham Heart Study [3], TCF7 expression was linked to increased lifespan and reduced cardiovascular risk. TCF7 is also highly evolutionarily conserved: it is one of only four genes consistently conserved in T cells across species [4].
“Our work shows that immune resilience is associated with TCF7, a central master regulator that maintains T cell health,” said Muthu Manoharan, MS, co-first author and senior research scientist at UT Health San Antonio.
The researchers view IR as a health-promoting (salutogenic) trait that protects against diseases and aging and can be targeted to increase healthy longevity. “When salutogenesis declines and pathogenesis emerges, this may create a state of inflammation and immune aging that promotes disease,” explained Sunil K. Ahuja, MD, professor in the department of medicine at the Joe R. and Teresa Lozano Long School of Medicine. “Individuals with TCF7-linked immune resilience appear better equipped to resist inflammatory stressors and maintain a low-inflammatory immune profile promoting survival and better health.”
The warranty period
Importantly, the researchers identified the period between ages 40 and 70 as the one where the differences between the three IR subtypes are most pronounced. People with low IR at 40 faced nearly tenfold higher mortality, equivalent to someone 15.5 years older with preserved immune resilience.
The resilience gap narrowed after age 70, as general systemic aging overtook the benefits of strong immunity. Per the researchers, this suggests a critical intervention window in midlife, when preserving or restoring IR could have the greatest impact. However, even beyond age 70, people with strong IR signatures continued to show molecular signs of better biological aging and some survival advantage.
Framing their findings within human evolution, the researchers proposed that immune resilience evolved to balance the benefits of inflammation with its long-term damage. This “biological warranty period,” as the authors call it, extending to around age 70, reflects the span during which IR offers a strong survival benefit. After that, the advantage diminishes as age-related pathologies accumulate. However, the more we know about how those processes shaped by evolution work, the better we can become at affecting them to promote health and longevity.
“We envision a future in which immune resilience is routinely assessed, much like cholesterol testing,” said Justin Meunier, BS, a bioinformatician at the Center for Personalized Medicine. “Optimal immune resilience is associated with a unique blood biomarker profile that reflects higher levels of growth and immune factors, along with lower levels of inflammation.”
“The study provides compelling evidence that immune resilience — not just the absence of disease — is a key determinant of longevity,” said Dr. David Furman, associate professor at the Buck Institute for Research on Aging, who was not involved in this study, to Lifespan.io. “This work highlights a critical reality: chronic inflammation, cumulative cell stress, and lifelong environmental exposures such as pollutants, diet, and lifestyle factors — what we call the exposome — are central drivers of aging. The exposome relentlessly interacts with our immune system, either preserving resilience or degrading it.”
“We’ve known for years that ‘inflammaging’ — the chronic, sterile inflammation that builds with age — is a core mechanism behind nearly every age-related disease,” Furman explained. “This paper elegantly frames healthy aging as an active, dynamic process. The implications are clear: strengthening immune resilience could be one of the most powerful and actionable strategies we have to extend healthspan, especially as we increasingly recognize that aging is shaped not only by our genes, but by the totality of our lived experiences and exposures.”
While the study controlled for major confounders like age, sex, and comorbidities, it remains observational and cannot fully account for unmeasured factors such as lifestyle or medication use. Experimental validation of TCF7’s role, which was not performed in this study, will certainly be needed.
Literature
[1] Manoharan, M. S., Lee, G. C., Harper, N., Meunier, J. A., Restrepo, M. I., Jimenez, F., Karekatt, S., Branum, A. P., Gaitan, A. A., Andampour, K., Smith, A. M., Mader, M., Noronha, M., Tripathy, D., Zhang, N., Moreira, A. G., Pandranki, L., Sanchez-Reilly, S., Trinh, H. D., . . . Ahuja, S. K. The 15-Year Survival Advantage: Immune Resilience as a Salutogenic Force in Healthy Aging. Aging Cell, e70063.
[2] Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., … & Slavich, G. M. (2019). Chronic inflammation in the etiology of disease across the life span. Nature medicine, 25(12), 1822-1832.
[3] Ho, K. K., Pinsky, J. L., Kannel, W. B., & Levy, D. (1993). The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology, 22(4), A6-A13.
[4] Jiao, A., Zhang, C., Wang, X., Sun, L., Liu, H., Su, Y., … & Zhang, B. (2024). Single-cell sequencing reveals the evolution of immune molecules across multiple vertebrate species. Journal of Advanced Research, 55, 73-87.








































