All:
Just seeing this now:
I just want to find out whether we should further waste our resources* on this substance e.g. try to replicate the data with our "Mprize@home" project or get some researchers to carry out some real aging-studies (maybe propose it for the NIA's Intervention Testing Program).
I think that the preliminary data on metformin makes it worthwhile to have it properly tested (see below), but I can't imagine that anyone's going to do so "@home": according to
this source, which is however for breeding females (which are doubtless somewhat more expensive than non-bred females, or males), teh cost to maintain a lab mouse is "$530/year (includes cost of technician, cage washing, bedding, feed, other supplies, but not the cost of genotyping", and to get statistically significant LS results requires more than 30 animals (more than 40 would be better, and more is better still) in both the control and the experimental goups to get a statistically significant results, so you're looking at something like US$37100 per annum, which you'd have to have dedicated for 4 y (or more, if the intervention is wildly successful) -- so, more than US$150 k. And as the number of lousy LS results that we've seen even in academic labs (where the "extension" is relative to a colony of otherwise long-lived mice that for reasons that are often unclear (tho' overfeeding is a common one) live shorter than they should) has shown us, even "real scientists" don't always do a credible job of animal husbandry, let alone we amateurs. And then, even if it really, unambiguously did work, the absence of formal institutional oversight (scientific and ethical) might lead to skepticism and/or hostility about the research and its results, delaying peer-reviewed publication, replication, and ultimate human translation for a difficult-to-anticipate period of time.
I hope you are not dismissing putative CR-mimetics, just because you are already on CR (is this even a conflict of interest per se?) I believe you don't, but I like to point out possible sources of bias regardless.
I don't see why you'd think of my CR practice would bias me
against a mimetic: if anything, being sufficiently convinced that rodent CR data will translate into human results ought to make me about as bullish as anyone can be of the benefits of such an agent, and having a pill would really save me a lot of time and hassle. Indeed, if I was against doing it in a pill, I'd probably have a problem with SENS, too. No, I spelled out my reasons for thinking the mimetic approach is going nowhere in (3) and (4), to some extent in Ending Aging, and in more detail in an unpublished MS from which an extract was posted privately
here (I believe that this is closed to all but Imminst Directors & Advisors, and ask people not to cross-post it to any public forum or otherwise circulate it in order to avoid impeding ultimate publication). To quote from (3):
Such agents are, first, likely to have unacceptable side-effects, as they will of necessity induce the dramatic metabolic shift which underlies both the desired and the undesired effects of CR. This is as we would expect from evolutionary theory (Kirkwood and Austad [ref]) . The signaling pathways in which such agents would intervene are normally set to maximize reproductive fitness, even at the expense of long-term somatic health, and thus exert pleiotropic effects on the health of the organism. If these pathways could be manipulated to retard our rate of aging at no cost to the “normal,” apparently “healthy” functioning of the organism within the parameters of our existing genetic toolkit, such adjustments would already have been made, as they would increase reproductive fitness. ...
Secondly, CR mimetics will be extraordinarily difficult to license for clinical use in humans, not only because of the regulatory hurdles facing a pharmaceutical designed to treat “aging,” but because they will (like CR itself) only retard the further accumulation of aging damage rather than reversing it. Prevention, rather than treatment, of age-related disease is by its nature a longer-term clinical endpoint. Thus, even extrapolating from the surprisingly positive results of Spindler’s group’s latest work in older animals (Dhahbi et al [ref]), any such drug will take many times the length of time usually required of existing pharmaceuticals to reveal clinically-significant benefits on “licensable” indications in its users.
Indeed, there is a definite irony to the fact that, as essentially preventive agents, CR mimetic drugs would at once be most likely to benefit patients if administered while they are still young and healthy, and yet be all the more difficult prove effective for any clinical outcome in such patients. And while the multi-decade efficacy trials proceed, such subjects will yet be forced to endure the great majority of the drug’s side-effects. Ethical review panels will be especially wary of approving clinical trials using agents whose safety and efficacy will not be clear for decades in young, healthy subjects. Thus, the time lag will be even greater before the full benefits of a true CR mimetic, as envisioned eg. by Richard Miller [ref], would be manifested in the clinic, in patient lives, and in reduced burden on public and private medical systems.(3)
The first point above is mathematically modeled in (5), with contradistinction to a damage-removal, "engineering"/SENS approach:
 Figure 1. Blue line: therapy that reduces the rate of damage accumulation by 50% without removing pre-existing damage. Green line: effect of progressively doubling the efficacy of such an intervention every 7 years. Pink line: "engineering"/SENS therapy that removes half the damage. Orange line: progressive refinement of such a therapy to remove half the damage that it previously could not remove every 20 y. From (5).
Figure 1. Blue line: therapy that reduces the rate of damage accumulation by 50% without removing pre-existing damage. Green line: effect of progressively doubling the efficacy of such an intervention every 7 years. Pink line: "engineering"/SENS therapy that removes half the damage. Orange line: progressive refinement of such a therapy to remove half the damage that it previously could not remove every 20 y. From (5).* I am not talking about diverting resources from basic research into aging and SENS, the most important piece of the puzzle. Just using available resources.
I think you mean vice-versa, right? Either way: dude, it's all one bathtub ...
Assuming I read this (from sci.life-ex) correctly, as I skimmed some of the discussion, there was another supposed life-extension report by Roth et al from the “Symposium on Neurobiology and Neuroendocrinology of Aging”, both the symposium and your post on the topic date back to 2002. I'm wondering what happened? Do you still consider their work to be legitimate? Does it merit further investigation?
I'm sorry that you only saw that post ... The study turned out to be a disappointment. Ingram et al never published the results, and the problem on that fuzzy survival curve:
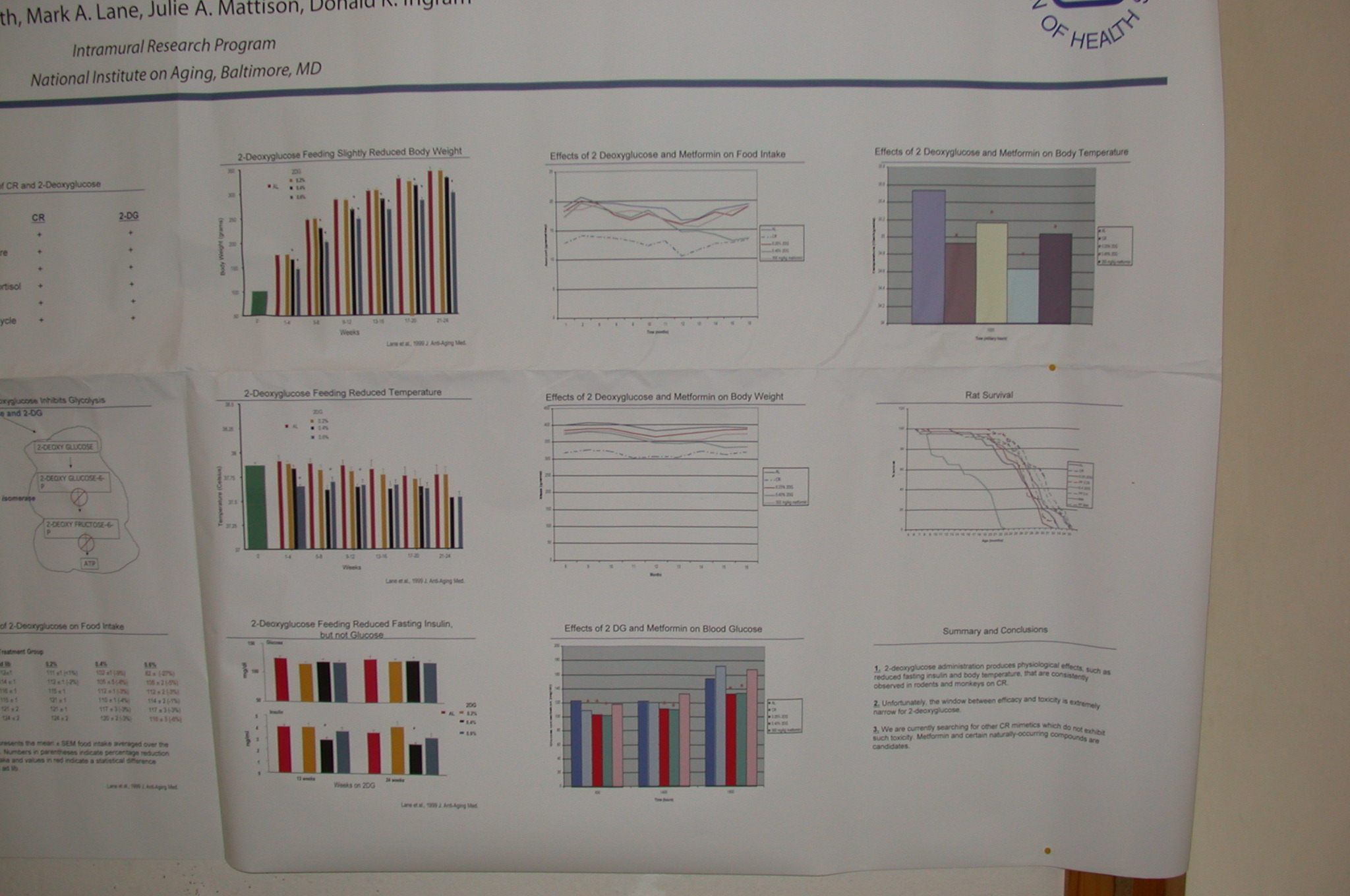
... is of course that you can't see the the #$(&*#!! X-axis (even on the
closeup), allowing one to project one's hopes into it. However, Ingram also presented the results informally at
SENS1/IABG10 (6), which I was privileged to attend (psst:
SENS4 is coming up -- show up for the thrill of the year!). It turns out that Metformin was one of several agents being given a very "quick"-and-dirty LS screen, and gave the clearest extension of LS: relative to the controls, there really is a significant extension of max LS, which LOOKs just like a CR curve. The bad news (wait for it!): the control group was short-lived. From my notes, written down unfortunately a week or so AFTER the event, and based on eyeballing the survival curves, the single longest-lived mouse (not max LS, which is operationally the av'g LS of the longest-lived 10%, to avoid outlier effects) of metformin vs control were 36 and 33 mo, respectively (and it might've been 33 and 30; Dr. Ingram said he'd send me a copy of the graph, but then decided he didn't want to risk violating publication embargo) ). 36 mo ~1096 days -- which is not too far from what a well-cared-for, well-nourished, non-fuckup rat lives in the lab anyway. I don't know what strain of rat this was, but eg. the AL sedentary Long-Evans rats used by Holloszy had a max LS clearly in excess of 1000 d (he doesn't give hard data in (7), but see his survival curve in Figure 2), and the max LS for F344 rats in the Biomarkers of Aging Program had max LS of: AL-F, 1072 (35.73 months); CR-F, 1292 (43.07 months); AL-M, 896 (29.87 months); CR-M, 1222 (40.73 months) (8). If they were using male F344 this might be promising -- but Ingram (and another source with close contact to his lab) confirm that there were problems with the protocol and the result can't be considered robust.
Also, there is the mortality data you mention, with the caveats you also mention: a modest superiority to other antidiabetic agents
in diabetics isn't exactly an overwhelming endorsement.
Overall, I'd say metformin's merits are more "meh" than 'mazing (yes, the alliteration is an intended conceit

); still, it's worth a followup -- just not a breathless one.
-Michael
References[1] Am J Med Sci. 2008 Sep;336(3):241-7.
Effect of metformin-containing antidiabetic regimens on all-cause mortality in veterans with type 2 diabetes mellitus.
Gosmanova EO, Canada RB, Mangold TA, Rawls WN, Wall BM.
[2] Arch Intern Med. 2008 Oct 27;168(19):2070-80.
Cardiovascular outcomes in trials of oral diabetes medications: a systematic review.
Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, Marinopoulos SS, Feldman L, Vassy J, Wilson R, Bass EB, Brancati FL.
Full:
http://archinte.ama-...ull/168/19/2070 3. Rae MJ.
You don't need a weatherman: famines, evolution, and intervention into aging.
AGE. 2006 March;28(1):93-109.
No PMID assigned yet.
4. Rae M.
It's never too late: calorie restriction is effective in older mammals.
Rejuvenation Res. 2004 Spring;7(1):3-8. Review. No abstract available.
PMID: 15256039 [PubMed - indexed for MEDLINE]
5. Phoenix CR, de Grey AD.
A model of aging as accumulated damage matches observed mortality patterns and predicts the life-extending effects of prospective interventions.
AGE. 2007 Dec; 29(4):133-189.
6. Ingram DK.
Development of calorie restriction mimetics as a prolongevity strategy.
Biogerontology. 2003;4(Suppl 1):45(Abs81).
7. Holloszy JO.
Mortality rate and longevity of food-restricted exercising male rats: a reevaluation.
J Appl Physiol. 1997 Feb;82(2):399-403.
PMID: 9049716 [PubMed - indexed for MEDLINE]
8. Holmes DJ.
F344 Rat. Inbred Rodent Resource.
Science of Aging Knowledge Environment (SAGE KE). 2003 Sep 10;36: as2.
Edited by Michael, 10 February 2009 - 11:52 AM.














































