This is going to be a lot of random blathering and a ton of text by me, so please bear with it if you are really interested. I just needed to get all my thoughts and data out and recorded somewhere. Others thoughts are always appreciated.
For some time I've been trying to ascertain how resveratrol activates Sirt1. Using the Fluor de Lys fluorescent system, it was thought resveratrol directly interacted with Sirt1, but this was later proved false, and an artifact of the fluorescent tag. So, if resveratrol does not directly interact with Sirt1, how is it activating it?
If anyone here has followed my discussions on niacinamide with 100YearsToGo, which can be found here, you would see how we've discovered, from this paper especially, that the usual levels of niacinamide in the cells are always high enough to inhibit Sirt1. There has been some talk on these forums about avoiding niacinamide supplementation due to its Sirt1 inhibitory effects, but this is not necessary since Sirt1 is always inhibited, except for its vital DNA repair role. Inhibition of Sirt1 is only ended when the ratio of NAD+ to niacinamide increases above the basal levels cells are set at. This happens in response to stressors, such as calorie restriction. CR and other certain types of stress turn on Nampt (also known as PBEF). Nampt is the enzyme that converts niacinamide back into NAD+, which turns on Sirt1 once there is more NAD+ to bind in comparison to niacinamide to inhibit. Could it be then, that resveratrol's real target isn't Sirt1, but Nampt, and that activation of Nampt is what turns on Sirt1? That is, that Nampt is the start of the whole cascade?
This paper, "Extension of Human Cell Lifespan by Nicotinamide Phosphoribosyltransferase" clearly points to this possibility. Nampt levels fall with aging, and inhibition of Nampt leads to early aging and senescence. Conversely, overexpression of Nampt increased Sirt1 activity by over 80%, Sirt1 protein abundance, oxidation resistance, and increased life span of vascular cells considerably. Using a dominate negative mutant of Sirt1 co-expressed in Nampt expressing cells resulted in the same phenotype as Nampt inhibition. So then, Nampt's effects are carried out by Sirt1. But, what about resveratrol?
This is all interesting because of this paper still in print that I just ran into, which I will not go into gory detail over. "Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol" by Subhendu Mukherjee et al. All data belongs to them, and is credited to their labs alone, and I'll remove this data if they don't want it up elsewhere from their paper. I do not want to infringe on any copyrights or intellectual property.
Actually getting the paper, we see some startling results, which I will post here for everyone (as I know many cannot get it). This is with rats fed 2.5mg/kg of resveratrol, and looking at their hearts after 14 days on indicated diets.
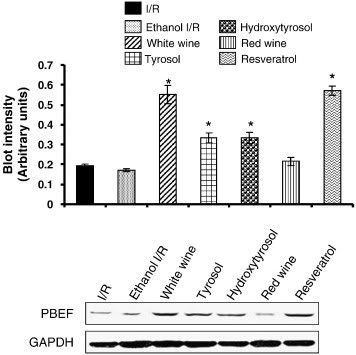
"Fig. 1. Western blot analysis of PBEF in heart tissue obtained from control (I/R) and EtOH-, white wine-, tyrosol-, hydroxytyrosol-, red wine-, and resveratrol-treated heart. GAPDH was used as a loading control. Figures are representative images of three different groups. Results are expressed as mean ± SEM of 3 groups of heart/group. low asteriskp < 0.05 vs Ethanol I/R."
Nampt (PBEF) is greatly increased in protein levels by resveratrol verses controls (I/R), by nearly a factor of 3. These aren't mRNA levels, which do not always mean protein is being transcribed, but actual protein. Interestingly, resveratrol's little brothers tyrosol and hydroxytyrosol can also activate Nampt, but not nearly as much (I call them such since they are structurally one half of resveratrol's structure). So we see immediately that resveratrol is causing overexpression of Nampt. Yes, it seems that Nampt may be its real target.
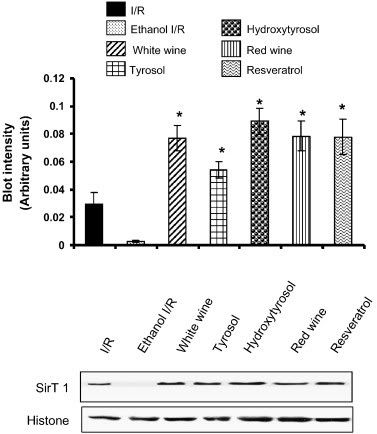
"Fig. 2. Western blot analysis of SirT1 protein in heart tissue obtained from control (I/R) and EtOH-, white wine-, tyrosol-, hydroxytyrosol-, red wine-, resveratrol-treated hearts. Histone was used as a loading control. Figures are representative images of three different groups, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM of 3 groups of heart/group. low asteriskp < 0.05 vs Ethanol I/R."
Resveratrol doesn't just activate Sirt1, it increases its protein expression in the heart, quite dramatically. Very interesting, as this falls in line with Nampt overexpression, which also upregulates Sirt1 expression. Also notice how the increase over controls is a little less than a factor of 2, again in line with what's been seen before that Nampt overexpression increases Sirt1 by about this much - though resveratrol could always be directly affecting these levels too. Hydroxytyrosol does it even better, however, as we'll see, since it does not increase Nampt (PBEF) nearly as much, hydroxytyrosol is not as potent or as protective as resveratrol. Still, it does imply that resveratrol may also directly activate Sirt1 translation, above just Nampt expression effects. This feeds directly in to the model of function I have come to conclude.
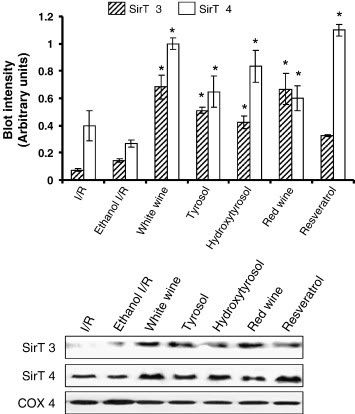
"Fig. 3. Western blot analysis of SirT3 and SirT4 protein in heart tissue obtained from control (I/R) and EtOH-, white wine-, tyrosol-, hydroxytyrosol-, red wine-, resveratrol-treated hearts. COX 4 was used as a loading control. Figures are representative images of three different groups, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM of 3 groups of heart/group. low asteriskp < 0.05 vs Ethanol I/R."
Ah yes, the other sirtuins, of which we have 7. These two are the mitochondrial sirtuins, unlike Sirt1 which is our nuclear version. What they do exactly is still being researched, but Sirt3 controls ATP production (loss of it decreases ATP made in mitochondria), and Sirt4 regulates insulin secretion (Sirt4 dysfunction may be part of the hyper insulin secretion in diabetes type 2). What Sirt4 does in the heart, however, is a big question. However, considering Sirt4 interacts with adenine nucleotide transporter (ANT), it's likely Sirt4 is important for the functions of ANT, which takes ATP out of the mitochondria to the rest of the cell, and brings in ADP to be converted to ATP, as Sirt3 is for Complex I. A critical transporter indeed. Futhermore, Sirt3 and 4 are required to stop the translocation of the apoptotic promoting factor from the mitochondria to the nucleus, and thus are vital proteins in activating the stress response and survival in conditions like CR. Again we see CR also activates Nampt expression, so everything ties together nicely.
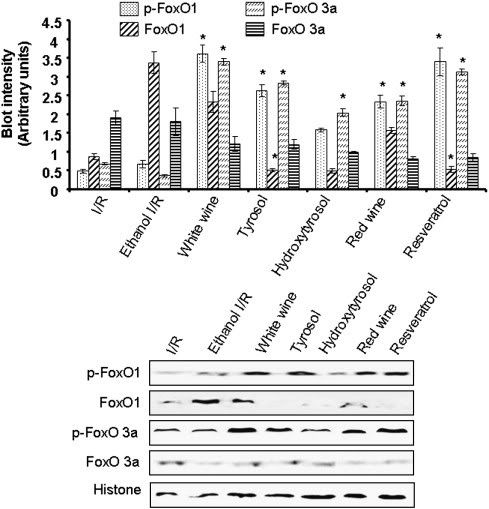
"Fig. 4. Western blot analysis of phospho-FoxO1, FoxO1, phospho-FoxO3a, and FoxO3a protein in heart tissue obtained from control (I/R) and EtOH-, white wine-, tyrosol-, hydroxytyrosol-, red wine-, resveratrol-treated hearts. Histone was used as a loading control. Figures are representative images of three different groups, and each experiment was repeated at least thrice. Results are expressed as mean ± SEM of 3 groups of heart/group. low asteriskp < 0.05 vs Ethanol I/R."
CR and Sirt1 activations leads to phosphorylation of FoxO1 and 3. These proteins, when dephosphorylated, tip the cell towards apoptosis the moment growth factors are removed from the media, but when phosphorylated, cause cell arrest and survival instead. Both functions are anti-cancer. At any rate, resveratrol greatly increases the phorsphorylated form of the FoxOs, protecting cells and activating survival. Phosphorylated FoxOs are important for longevity. This is in agreement with data on Nampt, which leads to AMPK activation through Sirt1, which then phosphorylates FoxOs.
Finally we have this table:
--------------------- I/R ------------- Resveratrol
Infarct size ------ 37 ± 2 ---------- 20 ± 1low asterisk
Apoptosis ------ 38 ± 2 ---------- 14 ± 2low asterisk
(most data removed to keep table manageable, but resveratrol had lowest infarct size, and second lowest apoptosis after hydroxytyrosol)
Resveratrol significantly decreases damage to the heart when infarcted (lack of blood flow, aka heart attack or heart disease).
It is so interesting to see such dramatic changes caused by resveratrol in the protein levels of Nampt and Sirt1. Increasing Nampt is where the model of resveratrol action comes in (see here). Since resveratrol increases Nampt, this allows conversion of niacinamide into NAD+. The increasing ratio of NAD+ to niacinamide leads to activation of Sirt1, which turns on PGC-1alpha, the master mitochondrial biogenesis regulator, AMPK and subsequent phosphorylation of FoxOs, and more.
We turn back to that question I posed under the thread title. Will niacinamide supplements stop resveratrol from turning on Sirt1? The answer, from this data, is possibly no. Not only maybe no, but they might actually increase the health benefits of resveratrol by giving Nampt more niacinamide to convert into NAD+, pushing the cell more towards NAD+ over NADH as well. Nampt is the rate limiting step in the conversion of niacinamide to NAD+, so the ratio is directly controlled by the speed Nampt starts the NAD+ synthesis pathway verses NAD+ being utilized back into niacinamide (by Sirt1 for instance). Having more Nampt, or more active Nampt, will tip the scales towards NAD+ if all other NAD+ utilizing factors stay the same (the increase in Sirt1 means it'll be self limiting and only work in proportion to Nampt), as we've seen with Nampt overexpression, which resveratrol simulates. So then, as long as there is enough Nampt, and it stays on long enough to tip the balance towards NAD+ and away from niacinamide, Sirt1 will become active.
However, if there is a great excess of niacinamide as through supplementation, resveratrol activated Nampt may not be on long enough due to resveratrol clearance and Nampt proteolysis, and the NAD+/niacinamide ratio may never rise high enough to activate Sirt1. We see this with in vitro muscle cells, here, where when resveratrol is added to cells after niacinamide, AMPK and glucose uptake are not activated, which is what they were measuring for Sirt1 activity (and consequently adequate Nampt activity). What happened to Nampt, or PGC-1alpha? Unfortunately they did not test these proteins, so we are left with a incomplete picture. Could it be addition of niacinamide before resveratrol was the reason resveratrol's effects were stopped?
Niacinamide without resveratrol has been shown to downregulate Sirt1 expression (here), and increase adipocity (fat). Apparently, niacinamide doesn't actually turn on Nampt, despite it being Nampt's substrate. However, there may be some tipping point where an overabundance of niacinamide does activate Nampt through other cellular watch dogs. However, what are those controllers of Nampt expression? That is, how did resveratrol increase Nampt expression? Is there an even more upstream target that resveratrol is really working on, or does Nampt feed back on itself for activation? Actually, it seems that Nampt might actually feed back on itself. For instance, Nampt activity activates AMPK, and this activation of AMPK is inhibited by niacinamide (see here again). Yet, on the other hand, AMPK activity is required to activate the transcription of Nampt in response to glucose restriction. Here we have a chicken or the egg problem. Does AMPK activate Nampt which activates AMPK, or does Nampt activate Sirt1 which activates AMPK, which then activates more Nampt production? The latter is what fits the evidence best in my opinion. However, it's still possible resveratrol is actually turning on AMPK. So then, if resveratrol acted directly to activate Nampt, that activity would snow ball into an increase in Nampt and efficient processing of niacinamide into NAD+. On the other hand, a high enough amount of niacinamide will inhibit AMPK and break this process. If resveratrol turned on AMPK which then turned on Nampt, niacinamide wouldn't be expected to break the process and stop AMPK activation, as resveratrol already would have done it. Let's look at this another way and ask what other ways are Nampt turned on?
Apparently, as stated before, Nampt transcription is activated by stress and CR, via such factors as IL-1beta (see here) and other proinflammatories. Since resveratrol is an anti-inflammatory molecule, it's not very likely that resveratrol is acting on higher inflammatory responsive pathways to turn on Nampt. Moreover, resveratrol activated Sirt1 actually protects from NF-kB activation, while IL-1beta causes damage, so it's unlikely NF-kB, which is the main responder to proinflammatory signals, turns on Nampt, and neither does IL-1beta in the same way. Even if IL-1beta upregulates Nampt mRNA, other pathways turned on by IL-1beta may outweigh it, or perhaps IL-1beta leads to Nampt secretion into the media instead of increase intracellularly.
Now we've moved into the realm of pure speculation and need to stop here, as I don't have the papers to address these questions. More research must be done, but it seems that for now, until higher targets in the pathway are found, I feel, and this is only my opinion, we can say resveratrol's real target is possibly Nampt, and only Sirt1 by consequence.
And niacinamide supplementation? Most likely this is favorable and only needs to be spaced a few hours after taking resveratrol, so that resv has the chance to upregulate Nampt protein levels. I would say three hours apart would be optimal, as resveratrol will be mostly cleared by then, and expression of Nampt should be in full swing, or already declining, and Sirt1 should have done its stuff. This could allow synergism between resveratrol and niacinamide, as there'll be plenty of Nampt around after resveratrol stimulation to take that niacinamide and convert it. We'd have to have kinetic studies looking specifically at these factors to know for sure.
That is, if resveratrol directly activates Nampt which turns on AMPK through Sirt1, which then turns on more Nampt, then adding niacinamide after this initial step is complete, while capable of inhibiting Sirt1 at first, should still end with the net induction of Sirt1 once Nampt clears the ratio back in favor of NAD+ - until basal levels are reached again. However, too much niacinamide would likely last longer than the enhanced Nampt activity, and indeed inhibit Sirt1 despite resveratrol enhancement - hence why a time spacer is needed between resveratrol intake and niacinamide intake. As niacinamide may stop expression of Nampt after some concentration, especially relative to resveratrol, similar to how over abundance of glucose drives NAD+ levels towards NADH and insulin response inhibition, which seems strange when insulin is trying to turn on clearance pathways for glucose - indeed a possible analogy to niacinamide and Nampt. Obviously, under normal conditions, Sirt1 itself will build up niacinamide, and Nampt will slowly dwindle to some low constant level, leaving us with the condition our well fed selves are at, where Sirt1 is consitutively inhibited and Nampt is functioning at reduced levels only enough to keep cells alive, but not start the stress response longevity pathways.
Now, one could say since Sirt1 activates AMPK, which activates Nampt, that resveratrol is still truly acting directly through Sirt1. The only problem here is that niacinamide is still around at inhibitory levels, and it takes Nampt to change that. It's still possible, if resveratrol directly counters niacinamide inhibition at the protein level, of course, but no direct action of resveratrol on the Sirt1 protein has ever been seen, minus that fluorescent tag. So, while possible, it seems less likely than resveratrol working via Nampt activation first, at this time.
There is a lot we don't know, but the picture is getting much clearer, and we can start connecting together previously unrelated seeming research, that now we know is all connected through the same pathways. So, to sum it all up, in my opinion, based on what we currently know, the real model of resveratrol action is this:
Resveratrol activates Nampt above basal levels -> Nampt converts more niacinamide to NAD+, pushing the balance towards NAD+ and allowing Sirt1, 3, and 4 if not more to become activated -> Activated Sirt1 leads to activation of AMPK irregardless of the real AMP/ATP ratio levels -> AMPK regulates the production of more Nampt and Sirt1. End result: Resveratrol boosts Nampt protein levels, Sirt1 protein levels and activity, AMPK activity, and leads to the resulting survival, metabolism enhancement, mitochondrial biogenesis, bone density increase, aging reversal phenotypes seen in vivo from resveratrol administration.
Pathway ends when: Sirt1 and other NAD+ dependent activities pass a critical point and niacinamide is produced faster than NAD+, or more niacinamide is ingested into the system and overwhelms Nampt activity -> Sirt1 becomes inhibited again -> AMPK becomes dephosphorylated -> Nampt is degraded -> NAD+/niacinamide ratios return to basal levels. Additionally, factors that activate AMPK dephosphorylation could break this cycle too, irregardless of niacinamide.
Edited by geddarkstorm, 27 January 2009 - 06:35 AM.





















































