Rapamycin Increases Mice Longevity
#31
Posted 10 July 2009 - 03:07 AM
#32
Posted 10 July 2009 - 07:00 AM
The drug in question turns out to be Rapamycin, and a few seconds of searching turned up an article on the subject.
http://www.nature.co...ature08221.html
If im reading this right, there is no reason younger mammals shouldnt benefit, its just for research purposes that treatment is only started late.
If this turns out to be true, it just might be a solid step towards escape velocity.
Ofcourse theres a way to go yet before its safe for humans, since its also a powerful immuno suppressant drug... And it would be such a bother having to live in a plastic bubble.
Edited by ArgusEritaramis, 10 July 2009 - 07:25 AM.
#33
Posted 10 July 2009 - 03:36 PM
It does seem as if excessive protein formation can be bad. In the case of cardiac hypertrophy leading to congestive heard failure it is especially bad. here could be an optimum rate of protein synthesis, not to much, not too little. Also, the pathways involved with mTOR are multiple, interrelated and complex. There are mTOR variants that are not inhibited by rapamycin which inhibitory effect is solely on the mTORC1 protein and not on the mTORC2 protein. This can lead to a hyper-activation of the kinase AKT via inhibition on the mTORC1 negative feedback loop while not inhibiting the mTORC2 positive feedback to AKT. This AKT elevation can lead to longer survival in some cell types. It is going to take a while for this to be unraveled, but I am sure we will find out more in the coming year.That's what I wonder about. I mean - if protein turnover is slowed - it may be not so good for other processes. Though it seems the body mass didn't change during the trial, what's even more interesting. Keeping the protein synthesis, but still inducing autophagy? Is that even possible?
Patience. we will soon know more.
Edited by maxwatt, 10 July 2009 - 03:41 PM.
#34
Posted 10 July 2009 - 03:46 PM
That's what I wonder about. I mean - if protein turnover is slowed - it may be not so good for other processes. Though it seems the body mass didn't change during the trial, what's even more interesting. Keeping the protein synthesis, but still inducing autophagy? Is that even possible?
i thought this was the entire point of autophagy. autophagy begins in as little as 2 hours after you eat.
when post-mitotic cells accumulate misfolded proteins (the basis of diseases like alzheimers, parkinsons, etc.), autophagy helps recycle these 'junk' proteins into useful proteins. this process allows protein synthesis to occur without an outside protein source.
#35
Posted 10 July 2009 - 05:14 PM
The mamalian TOR target has two complexes. mTorc1 and mTorc2. It appears that Rapamacyn does not inhibit mTOR completely. It only inhibits mTorc1 and only partially.
These guys developed torin1 which they claim inhibits mTorc1 and Torc2 to a far greater degree. That would result in better tumor treatment among other things. What would it do for longevity? I wonder.
If you want to order the stuff to tests it on a bunch of aging mice...go here.
Edited by 100YearsToGo, 10 July 2009 - 05:17 PM.
#36
Posted 10 July 2009 - 05:33 PM
i thought this was the entire point of autophagy. autophagy begins in as little as 2 hours after you eat.
when post-mitotic cells accumulate misfolded proteins (the basis of diseases like alzheimers, parkinsons, etc.), autophagy helps recycle these 'junk' proteins into useful proteins. this process allows protein synthesis to occur without an outside protein source.
Rapamycin is an excelent inducer of autophagy in yeast, but a very poor one in mamals. So I doubt the age defying effect is because of autophagy.
Edited by 100YearsToGo, 10 July 2009 - 05:37 PM.
#37
Posted 10 July 2009 - 06:00 PM
Rapamycin is an excelent inducer of autophagy in yeast, but a very poor one in mamals. So I doubt the age defying effect is because of autophagy.
then, i guess the longevity response of rapamycin is more closely tied to the immuno-suppressive effect. the ROS reduction is probably the central issue here.
Edited by prophets, 10 July 2009 - 06:02 PM.
#38
Posted 10 July 2009 - 06:13 PM
then, i guess the longevity response of rapamycin is more closely tied to the immuno-suppressive effect. the ROS reduction is probably the central issue here.
How would that be a good thing for people especially the elderly with an already weakening immune system?
#39
Posted 10 July 2009 - 07:09 PM
Can you say anything about the relative rates? Below is the abstract you linked above. If I'm reading it correctly with my virus-addled head, you are right that autophagy isn't it. If so, that's rather huge. I was all set to think of it as a CR mimetic... Not sure it isn't, but, well, these are exciting times.Rapamycin is an excelent inducer of autophagy in yeast, but a very poor one in mamals. So I doubt the age defying effect is because of autophagy.
An ATP-competitive Mammalian Target of Rapamycin
Inhibitor Reveals Rapamycin-resistant Functions
of mTORC1
Received for publication, January 15, 2009 Published, JBC Papers in Press, January 15, 2009, DOI 10.1074/jbc.M900301200
Carson C. Thoreen, Seong A. Kang, Jae Won Chang, Qingsong Liu, Jianming Zhang, Yi Gao,
Laurie J. Reichling, Taebo Sim, David M. Sabatini, and Nathanael S. Gray
The mammalian target of rapamycin (mTOR) kinase is the
catalytic subunit of two functionally distinct complexes,
mTORC1and mTORC2, that coordinately promote cell growth,
proliferation, and survival. Rapamycin is a potent allosteric
mTORC1inhibitor with clinical applications as an immunosuppressant
and anti-cancer agent. Here we find that Torin1, a
highly potent and selective ATP-competitive mTOR inhibitor
that directly inhibits both complexes, impairs cell growth and
proliferation to a far greater degree than rapamycin. Surprisingly,
these effects are independent of mTORC2 inhibition and
are instead because of suppression of rapamycin-resistant functions
of mTORC1 that are necessary for cap-dependent translation
and suppression of autophagy. These effects are at least
partly mediated by mTORC1-dependent and rapamycin-resistant
phosphorylation of 4E-BP1. Our findings challenge the
assumption that rapamycin completely inhibits mTORC1 and
indicate that direct inhibitors of mTORC1 kinase activity may
be more successful than rapamycin at inhibiting tumors that
depend on mTORC1.
#40
Posted 10 July 2009 - 08:20 PM
Can you say anything about the relative rates? Below is the abstract you linked above. If I'm reading it correctly with my virus-addled head, you are right that autophagy isn't it. If so, that's rather huge. I was all set to think of it as a CR mimetic... Not sure it isn't, but, well, these are exciting times.
I'm not 100% sure either. I said I doubt it. From the study i posted:
"Moreover, in yeast rapamycin strongly promotes induction of autophagy (self-eating), a process by which cells consume cytoplasmic proteins, ribosomes, and organelles, such as mitochondria, to maintain a sufficient supply of amino acids and other nutrients (10). The effects of rapamycin in mammalian cells are similar to those in yeast, but typically much less dramatic and highly dependent on cell type. For instance, rapamycin only causes cell cycle arrest in a limited number of cell types and has modest effects on protein synthesis (11–13). Moreover, rapamycin is a relatively poor inducer of autophagy, and it is often used in combination with LY294002, an inhibitor of PI3K and mTOR (14)"
11. Neshat, M. S., Mellinghoff, I. K., Tran, C., Stiles, B., Thomas, G., Petersen, R., Frost, P., Gibbons, J. J., Wu, H., and Sawyers, C. L. (2001)
Proc. Natl. Acad. Sci. U. S. A. 98, 10314–1031912. Pedersen, S., Celis, J. E., Nielsen, J., Christiansen, J., and Nielsen, F. C. (1997) Eur. J. Biochem. 247, 449–456
13. Shor, B., Zhang, W. G., Toral-Barza, L., Lucas, J., Abraham, R. T., Gibbons, J. J., and Yu, K. (2008) Cancer Res. 68, 2934–2943
14. Takeuchi, H., Kondo, Y., Fujiwara, K., Kanzawa, T., Aoki, H., Mills, G. B., and Kondo, S. (2005) Cancer Res. 65, 3336–3346
The last reference (14) gives contains an indication of how poor an inducer it is. I have not been able to access it (for free) yet.
#41
Posted 10 July 2009 - 08:39 PM
#42
Posted 11 July 2009 - 12:31 AM
How would that be a good thing for people especially the elderly with an already weakening immune system?
What popped into my head immediately is that the negative effects of chronic inflammation are being reduced through the immuno-supressive effects of rapamycin. I am just layman, but just following the science over the last few years it seems chronic inflammation is one common aspect in aged mammals. The immune system reacts to all the broken down malfunctioning cells and organs throwing off massive inflammatory signals. This misplaced auto-immune attack amplifies aging processes and leads to systemic failures and death. Rapamycin tames the immune response. Feel free to slap me if I am way off-base here. I guess there would be a trade-off with rapamycin treatment. As mentioned earlier, a person might become more susceptible to infectious disease.
#43
Posted 11 July 2009 - 01:26 AM
Would Rapamycin make one more susceptible to infections at the dose required to have a positive life lengthening effect ?
Has it been shown that current Rapamycin use has been part cause to increased infection rates ?
The immune system is still not understood, what I mean is..unless we can answer the questions above..we wont know for sure if this is an issue.
It maybe immunosuppressive..but does it act on the whole of the immune system..or is it selective at what it suppresses ?
Selectively blocking certain cell cummunications can allow the immune system to function whilst at the same time suppress Self Inflammatory responses ..perhaps this is what we need...to stop Autoimmunity and lengthen life....
Is Rapamycin it ?
#44
Posted 11 July 2009 - 03:50 AM
Th immune system is not understood fully...therefore
Would Rapamycin make one more susceptible to infections at the dose required to have a positive life lengthening effect ?
Has it been shown that current Rapamycin use has been part cause to increased infection rates ?
The immune system is still not understood, what I mean is..unless we can answer the questions above..we wont know for sure if this is an issue.
It maybe immunosuppressive..but does it act on the whole of the immune system..or is it selective at what it suppresses ?
Selectively blocking certain cell cummunications can allow the immune system to function whilst at the same time suppress Self Inflammatory responses ..perhaps this is what we need...to stop Autoimmunity and lengthen life....
Is Rapamycin it ?
If only it were that simple. While the mice in the study were protected in the laboratory, people taking rapamycin are very susceptible to life-threatening infections and cancers, and require constant medical supervision.
I've been looking for other mTOR inhibitors, there are a few: metformin. caffeine in high doses, resveratrol in near-toxic doses (in rodents, no idea how this might work for people) curcumin (but perhaps by a different pathway than rapamycin, so maybe yes, maybe no, and the high dose needed implies side effects.)
#45
Posted 11 July 2009 - 03:58 AM
Rapamycin is selective, but perhaps not selective enough. It is good enough that transplant rejection can be curtailed while other problems can be managed.It maybe immunosuppressive..but does it act on the whole of the immune system..or is it selective at what it suppresses ?
[...]
Is Rapamycin it ?
I suspect that rapamycin is not it, but that it points the way to a future it. I think the future it could prove to be seriously interesting.
#46
Posted 11 July 2009 - 07:15 AM
#47
Posted 11 July 2009 - 12:21 PM
I've been looking for other mTOR inhibitors, there are a few: metformin. caffeine in high doses, resveratrol in near-toxic doses (in rodents, no idea how this might work for people) curcumin (but perhaps by a different pathway than rapamycin, so maybe yes, maybe no, and the high dose needed implies side effects.)
Add glutamine to the list. I wonder, though each of these may require prohibitively large doses or have undesired side effects, if instead a combination would might have an additive effect without side effects? For instance, curcumin plus caffeine plus metformin? I am speculating, not recommending.
#48
Posted 11 July 2009 - 01:06 PM
i can try to put you in touch w/ him, if you like.
#49
Posted 11 July 2009 - 01:30 PM
Add glutamine to the list. I wonder, though each of these may require prohibitively large doses or have undesired side effects, if instead a combination would might have an additive effect without side effects? For instance, curcumin plus caffeine plus metformin? I am speculating, not recommending.
What I find interesting is that all those potential mTOR inhibitors you mentioned..are cited individually to help against inflammation...albeit with different published mechanisms (at least what is known)....perhaps one can say they are all related though by the fact they can all inhibit mTOR to some extent.?!..coincidence ...mTOR=Common Anti-Inflammation Mechanisim ??
Metformin - Perhaps helpful Diabetes/Glucose Intolerance/CNS
Caffeine - Pehaps Helpful to the Liver
Curcumin - Perhaps Anti-Inflammatory throughout the body
Glutamine - Used by some as an Anti-Inflammatory
Speculation..all speculation
Edited by youandme, 11 July 2009 - 01:37 PM.
#50
Posted 11 July 2009 - 02:00 PM
#51
Posted 11 July 2009 - 05:06 PM
Rapamycin may extend lifespan by postponing death from cancer, by retarding mechanisms of ageing, or both.
I don't have access to the actual paper, so I don't know why the authors made that statement, but I'd guess that the evidence seemed to suggest that protecting from cancer might be the major role played by rapamycin. Now, I don't know whether this is true or not, but in the resveratrol threads it was suggested that mice (or just a strain of mice?) seem to die overwhelmingly from some form of cancer -- more so, I gather, than human beings. Resveratrol, in contrast to rapamycin, does not seem to affect cancer rates, or at least not the form of cancer (lymphoma I believe) that seems to kill so many mice. This was offered up as a possible reason that resveratrol showed no life extension benefit when introduced in isolation.
So, can anybody answer the question: is there anything demonstrably wrong with the hypothesis that it's only rapamycin's and resveratrol's differing effects on cancer that gives them differing life extension benefits with mice? Is there anything demonstrably wrong with the hypothesis that resveratrol might actually be more effective for life extension in human beings?
Also, I have another unrelated question: why did the researchers choose the dosage of rapamyicn that they did? Why only one dosage? One wonders, of course, how the numbers might have been affected had the dosage been higher or lower. Might they have been much better with an optimally chosen dosage? Also, did the mice see any of the negative side effects of rapamycin that human beings do? If so, did they have any potential impact on rate of death?
#52
Posted 11 July 2009 - 05:07 PM
Oh, come on. Inflammation, just like cancer, or "boosting the immune system" is a fancy term devoid of any meaning. There are thousands of cancers, millions of ways to modulate the immune system and inflammation. Furthermore, no "anti-inflammatory" substance has ever demonstrated meanigful life extension and many have been tested -- this is so much bigger. All anti-inflammatory effects are probably downstream effects and if anything we would have to look for specific effects on specific types of inflammation.What I find interesting is that all those potential mTOR inhibitors you mentioned..are cited individually to help against inflammation...
There's simply no proof that inflammation plays a role in this, anti-inflammatory therapies like Aspirin always fail and very powerful interventions like ablation of visceral fat (which is pro-inflammatory itself) provide much smaller benefits than rapamycin. I suppose we could speculate, but I just don't see the biological plausiblity of mere systemic inhibition of inflammation.What popped into my head immediately is that the negative effects of chronic inflammation are being reduced through the immuno-supressive effects of rapamycin. I am just layman, but just following the science over the last few years it seems chronic inflammation is one common aspect in aged mammals. The immune system reacts to all the broken down malfunctioning cells and organs throwing off massive inflammatory signals. This misplaced auto-immune attack amplifies aging processes and leads to systemic failures and death. Rapamycin tames the immune response. Feel free to slap me if I am way off-base here. I guess there would be a trade-off with rapamycin treatment. As mentioned earlier, a person might become more susceptible to infectious disease.
http://mfoundation.o...amp;postcount=7
I haven't seen any evidence that immuno-suppression is necessary for rapamycin to work, which would be rather implausible, yet possible (and VERY unfortunate). I've actually never seen any evidence suggesting much of an involvement of immune-function in determining maximum life span and the rate of aging (I know that immuno-senescence plays some role in aging, though). Also I don't see a rationale for it, because inhibition of systemic ROS production has never produced meaningful life extension in rodents.then, i guess the longevity response of rapamycin is more closely tied to the immuno-suppressive effect. the ROS reduction is probably the central issue here.
The hypotesis postulated by the authors is much more plausible (at least at first glance): simple (be it partial or not) mTORC1 inhibition.
It would probably KILL you at the doses used (adjusted ~13mg in humans). If you are lucky, you would be just much more likely to die (primarily cancer and infection), like all the other people receiving transplants...Th immune system is not understood fully...therefore
Would Rapamycin make one more susceptible to infections at the dose required to have a positive life lengthening effect ?
....
Is Rapamycin it ?
Edited by kismet, 11 July 2009 - 05:25 PM.
#53
Posted 11 July 2009 - 05:16 PM
The dose was probably chosen based on mTORC1 inhibition and, no,"protection" from cancer isn't it. The authors note that it may "postpone death" (i.e. reduced cancer mortality), while in fact rapamycin seems to cause cancer in mice the same way it does in humans.I don't have access to the actual paper, so I don't know why the authors made that statement, but I'd guess that the evidence seemed to suggest that protecting from cancer might be the major role played by rapamycin.
...
Also, I have another unrelated question: why did the researchers choose the dosage of rapamyicn that they did? Why only one dosage? One wonders, of course, how the numbers might have been affected had the dosage been higher or lower. Might they have been much better with an optimally chosen dosage? Also, did the mice see any of the negative side effects of rapamycin that human beings do? If so, did they have any potential impact on rate of death?
In humans: http://www.rxlist.co...pamune-drug.htm
35 cancers in the rapamycin groupd vs 23 in controls, I don't think we can chalk this up to it's life extending effects (i.e. older mice get more cancers). Rapamycin is extremely dangerous and we are screwed if its effects are in any way tied to immuno-suppression.
http://www.nature.co...ure08221-s1.pdf
#54
Posted 11 July 2009 - 07:44 PM
I hope to post more on various issues later ... meanwhile:
- First, a big shout-out for Kismet for doing high-quality analysis on this thread!
Second: I'm sure those who've even perused the abstract will've noticed this very important fact, but for those who haven't and those who haven't yet dug further:
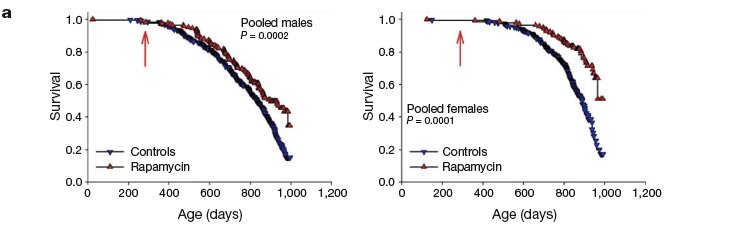
In a separate study, rapamycin fed to mice beginning at 270 days of age also increased survival in both males and females, based on an interim analysis conducted near the median survival point. …
At the time of analysis, 51% of the females and 68% of the males had died, and a stratified log-rank test showed significantly lower mortality risk in the rapamycin-treated mice compared to controls, pooling across the three test sites (P=0.0002 for males and P<0.0001 for females). When each site was evaluated separately, the beneficial effect of rapamycin for females was significant at each site (P,0.005); for males, the effect was significant (P<0.025) at UM and UT, but not at TJL. [MR: The 3 labs, unfortunately, used different diets early on, both between labs and (for UM and UT) between control and treated males, and this study and esp the previous report suggest that this affected later LS in the males; here, "We thus cannot rule out the possibility that improved survival among males in the rapamycin group, at UT and at UM, might reflect differences in nutritional or health status between control and rapamycin groups before 600 days, rather than solely the effects of rapamycin. Notably, the significant benefits of rapamycin on male (and female) survival at TJL could not have been affected by diet before drug administration, because at TJL both control and rapamycin-fed mice received the same chow (Purina 5LG6) throughout this period. "] Rapamycin seems to reduce mid-life mortality risk when started at 270 days of age, but additional data are needed to provide an accurate estimate of effect size, and to evaluate effects on maximal longevity.
Survival plots for male and female mice, comparing control mice to rapamycin-treated mice ... from 270 days of age. Because at the time of the interim analysis all live mice were between 800 and 995 days of age, we have only limited information about the shape of the survival curve at ages above 900 days, and the apparent change in slope at the oldest ages (.990 days) reflects this experimental uncertainty.
That certainly looks like there's going to be an extension of max LS -- but then, the same was true at the median point for NGDA in the first round ...
Another happy thing: even the stated data for mean and max LS extension for the late-onset animals likely slightly downplays the power of the result, as at the time of analysis not all of the animals had died:
This, I think, largely mitigates against the concern that it's only a cancer-preventive, since it doesn't appear that it was preventive. However, as Kismet notes, (a) there did actually look to be an increase in lymphoma in the treated group, and (b)they don't appear to give enough info to say whether there was a difference in age-specific incidence rates, or if more animals died of cancer simply because of a shift in causes of age-related death -- or, for that matter, if more treated animals died with, but not of, a higher burden of slow-growing, nonfatal tumors.We analysed the data set as of 1 February 2009, with 2% (38 of 1,901) of mice still alive. … Summing across the three sites, 4.8%of the female control mice were alive at these ages, compared with 21.5% of the rapamycin-treated females (P<0.0001). For males, the corresponding values were 5.9% of controls and 20.2% of rapamycin-treatedmice (P<0.0001). The site-specific calculations documented a significant effect on females at both TJL (P=0.0006) and UM (P=0.0001); for males, we noted a significant effect at both TJL (P=0.008) and UT (P=0.0001), with a marginal effect at UM (P=0.07). Rapamycin feeding initiated at 600 days of age thus leads to a significant increase in maximal lifespan.
To test if the spectrum of lesions was altered by dietary rapamycin, complete necropsies were conducted on 31 control and 40 rapamycinfed mice that were either found dead or killed when moribund (details in Supplementary Table 2). Although rapamycin postpones death, it did not change the distribution of presumptive causes of death.
Too much, in this thread, I fear. Folks, I implore you as friends and life extensionists not to fall prey to the temptation to leap from a possible mechanism to supplementation based on that mechanism (especially not in vitro!), especially not with very 'shotgun' supplements like curcumin, and especially not on the basis of the in vitro effects of those supplements. There are plenty of drugs that lower cholesterol or blood pressure, but kill you dead for other reasons.Speculation..all speculation
#55
Posted 11 July 2009 - 09:44 PM
http://www.popsci.co...d-easter-islandWhen tested on mice that had already reached middle age, the subjects treated with rapamycin increased their lifespan by 28-38 percent. Scientists are identifying these studies as the most promising drug-induced technique for increasing longevity, which is generally possible only via genetic manipulation or limiting caloric intake.
Edited by solbanger, 11 July 2009 - 09:47 PM.
#56
Posted 12 July 2009 - 02:13 AM
The dose was probably chosen based on mTORC1 inhibition and, no,"protection" from cancer isn't it. The authors note that it may "postpone death" (i.e. reduced cancer mortality), while in fact rapamycin seems to cause cancer in mice the same way it does in humans.
Not sure why you say rapamycin "causes" cancer in mice, if as Michael's post suggests, the basic breakdown in the number of deaths caused by cancer in the mice fed rapamycin is essentially equivalent to the number of such deaths in the controls (with possibly some greater proportion of lymphoma cancers among the dead mice).
My point simply reverts to the assertion the authors themselves made in the abstract, that
Rapamycin may extend lifespan by postponing death from cancer, by retarding mechanisms of ageing, or both.
I assume that there's something non-trivial the authors are trying to assert here, when they raise the possibility that the effects of rapamycin may, consistent with the evidence, be accounted for by assuming rapamycin is merely "postponing death from cancer". I assume that they may be asserting that there's a possibility that for some period of time rapamycin may protect from cancer (or at least death from cancer -- presumably the idea would be that the mice initially contract cancer at the same times as their peers in the control group, but don't so quickly succumb from it), and that the outcome might be fully explained under that assumption.
Now I certainly agree with you that for any of us to take rapamycin given its known side effects for human beings would be at this stage an act of madness.
But what I think we really need to find out is whether it's the cancer protective (i.e., cancer forestalling, or cancer retarding) properties of rapamycin that's giving us these numbers, or something more basic. In fact, given the apparent dominance of cancer in killing off all these mice, and of lymphoma in particular, one wonders how easy it might be to tease out the effects of forestalling cancers or death from cancer from the larger issue of extending life for these mice. How does one make that determination definitively? (Is it the case, for example, that the oldest mice even in the classic CR experiments, when they do finally die, don't do so from cancer but from some other cause? I assume there's some reason researchers conclude that it isn't simply putting off death from cancer that gives CR a legitimate claim to extending life. What should we be looking for from rapamycin that, apparently, the researchers here don't yet claim to have observed?)
And of course all this relates back to human beings, who don't seem so much to die from cancer (or at least I gather they don't), and certainly not from lymphoma in particular. If, say, resveratrol is a better protective against the causes that kill human beings (such as frail bones and muscles, cardiovascular weakness, etc.) it's still possible that human beings would respond better to resveratrol than to an mTOR inhibitor, if that mTOR inhibitor only forestalls cancer or retards death from cancer.
#57
Posted 12 July 2009 - 02:23 AM
Oh, come on. Inflammation, just like cancer, or "boosting the immune system" is a fancy term devoid of any meaning. There are thousands of cancers, millions of ways to modulate the immune system and inflammation. Furthermore, no "anti-inflammatory" substance has ever demonstrated meanigful life extension and many have been tested -- this is so much bigger. All anti-inflammatory effects are probably downstream effects and if anything we would have to look for specific effects on specific types of inflammation.
Thanks for replying to my post...I kept it loose 'Inflammation' for clarity...to highlight that all the substances listed act on Inflammation... perhaps I should have said 'differing Inflammation and with differing mechanisms'..apologies.
I would trade you a "million ways to modulate the immune system and inflammation" for one that works and works without side effects.
#58
Posted 12 July 2009 - 01:13 PM
....
Too much, in this thread, I fear. Folks, I implore you as friends and life extensionists not to fall prey to the temptation to leap from a possible mechanism to supplementation based on that mechanism (especially not in vitro!), especially not with very 'shotgun' supplements like curcumin, and especially not on the basis of the in vitro effects of those supplements. There are plenty of drugs that lower cholesterol or blood pressure, but kill you dead for other reasons.
Metformin has previously been touted as a life-extension aid. It does have potentially lethal but rare side effects.
Caffeine's side effects are known, particularly in large doses.
There is a large group of multiple myeloma patients taking as much as 8 grams of turmeric daily, for over four years. It seems to be a viable treatment for the condition. Some side effects are reported HERE . On balance, it seems to me the risks from curcumin supplementation are low enough to outweigh the possible unknown negatives. Especially if one is over sixty.
#59
Posted 12 July 2009 - 03:43 PM
Given that both CR and Rapamycin seem to work through inhibiting mTor, I don't see the harm in speculating on the mechanism involved. What I consider an "inflammatory response" is basically anything that involves the body responding to ROS, not necessarily a persistent condition of arthritis treated with aspirin. Running outside produces ROS and the body responds to it. If CR reduces MitROS output, such that the body sustains less damage. If Rapamycin retards protein synthesis and the body's response to ROS (even if it occurs), then there are some mechanistic parallels here, at the very least.
ill try to respond later more in depth.
#60
Posted 12 July 2009 - 04:02 PM
Kismet: There's simply no proof that inflammation plays a role in this, anti-inflammatory therapies like Aspirin always fail and very powerful interventions like ablation of visceral fat (which is pro-inflammatory itself) provide much smaller benefits than rapamycin. I suppose we could speculate, but I just don't see the biological plausiblity of mere systemic inhibition of inflammation.
http://mfoundation.o...amp;postcount=7
I haven't seen any evidence that immuno-suppression is necessary for rapamycin to work, which would be rather implausible, yet possible (and VERY unfortunate). I've actually never seen any evidence suggesting much of an involvement of immune-function in determining maximum life span and the rate of aging (I know that immuno-senescence plays some role in aging, though). Also I don't see a rationale for it, because inhibition of systemic ROS production has never produced meaningful life extension in rodents.
The hypotesis postulated by the authors is much more plausible (at least at first glance): simple (be it partial or not) mTORC1 inhibition.
Perhaps I didn't make it clear enough in my first post that I was talking about the immune system reaction to chronic inflammation. I was not talking about reducing inflammation but reducing the reaction to that inflammation. I was just speculating about the negative effect of the immune system responding to the chronic inflammation produced by age-related cellular damage. It is pure speculation on my part since like you, I am unaware of any studies showing maximal lifespan extension through immuno-supression. Most people would expect the opposite. Perhaps, in a controlled environment where death from infectious disease is extremely low, then some immune suppression in the aged body could be good.
2 user(s) are reading this topic
0 members, 2 guests, 0 anonymous users