All:
To reiterate what I said in the above post: I haven't seen but would appreciate evidence showing that (regular) PUFA meaningfully influences membrane unsaturation index or more importantly membrane peroxidation index.
... and more importantly
mitochondrial membrane peroxidizability index. As I said in a related context to Kismet, my apologies for leaving him (and the rest of you) you waiting ... I have a serious backlog of folks to whom I should be getting back. Part of the reason I am always falling behind is because I have an all-or-nothing perfectionist streak, which leads to a little bit of 'all' and far too much 'nothing' ... To get out SOMETHING, I'm going to largely just do a big link list, starting above all with asking folks to review the line of argumentation and the LIVE links in
this post and supporting studies (1-15)
here. The following studies then reconstruct those that haven't apparently been permanently lost (one of which, unfortunately, was the most important of all) and a couple of new ones. And, I also apologize because at least sme are also redundant to embedded links above. So please see:
MiRFAA: MiFR: Flax, not Fish? - sci.life-extension | Google GroupsMiFR: Flax, not Fish(Yes, those are 2 different posts)
crsociety : Message: [CR] DHA Causes mt Peroxidation (and related stuff on fats)"RE: Michael Rae's mitochondrian theory" post. Note the link takes you to a mishmash of 2 entirely different posts; to see the
relevant material,
SKIP DOWN TO the post by Dean P beginning with "Greg Watson wrote:".
Effects of Dietary Fatty Acids on Mitochondrial and Tissue Membrane Fatty Acid Composition & Peroxidizability Index.
Happily, albeit entailing additional work for me, 2 extremely useful studies, which I hadn't seen since last revisiting the issue, have provided some of the most comprehensive results yet. First is a study on the "
Effects of alpha-linolenic acid vs. docosahexaenoic acid supply on the distribution of fatty acids among the rat cardiac subcellular membranes after a short- or long-term dietary exposure. (1) Table 3 shows total "
Cardiac phospholipids fatty acid profiles (% of total fatty acids) from rats fed experimental diets for 2 or 6 months", and more importantly Table 6 shows
Mitochondrial fatty acid profiles. Rearranging that table to fit here:
Cardiac mitochondrial membrane phospholipid DHA (22:6 n-3) after 2 monthsControl diet 4.4 ± 0.28c
DHA diet 24.5 ± 0.97a
ALA diet 9.4 ± 0.25b
Cardiac mitochondrial membrane phospholipid DHA (22:6 n-3) after 6 monthsControl diet 3.4 ± 0.10c ***
DHA diet 20.4 ± 2.73a ***
ALA diet 8.2 ± 0.35b
(If it looks as if the control diet is better than the ALA diet, well, it is: the control diet provides only the minimum essential fatty acids (LA and ALA) for the rodent RDA, whereas the ALA and DHA diets are necessarily enriched. We want as little n3 and n6 as lowers disease risk, and can make up the rest of our dietary fat (and it's pretty clear that one should be getting ≥30% fat in the diet) as MUFA, which is even less desaturated (1 double bond) than ALA (3 double bonds) or LA (2)).
The results for total cardiac membrane phospholipids are parallel, albeit less important.
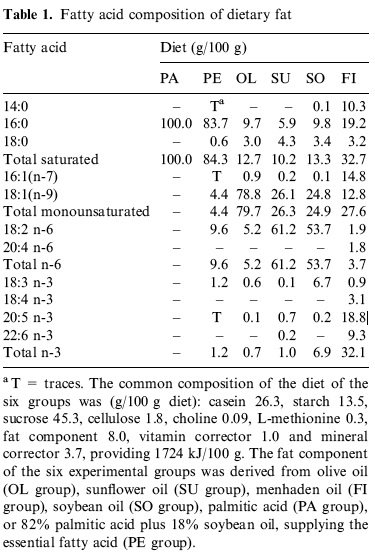
(2) covers the effects of a wider range of diets ("olive oil, sunflower oil, fish oil, soybean oil, palmitic acid, or 82% palmitic acid plus 18% soybean oil, supplying the essential fatty acid") on the
tissue membranes of several different tissues ("thymocytes, pancreatic exocrine, muscle and adipose tissues "), tho' we're mostly concerned with mt membranes and with postmitotic tissues like muscle; the results are harder to post, but give this a whirl:


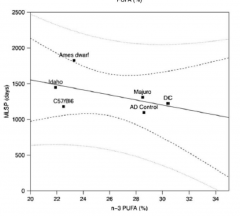
Hey! That works pretty well, so long as your vision is good ...
Again, I expect that the relatively small benefits of this will acrue to those who are getting up against the limits of human life expectancy, not the average Jo(e) on the street or even a very healthy non-CR person; it's likely important to CR so that you aren't overriding a known effect and likely mechanism if indeed CR works in humans, and it might also be important to people who either get lucky statistically, or have 'longevity genes.'
It is interested, though, that DHA seems to protect LDL from oxidation... (cite)
First, that's
in vitro oxidizability of LDL, which is totally irrelevant to the way that LDL is oxidized
in vivo: what you want to look at is the level of endogenously-oxidized LDL derived
in situ from blood samples. And second, even if you had that, it would be LDL oxidation -- our issue is
tissue (and, again, especially
mitochondrial)
membrane peroxidizability.
One would expect trans fats to do the same thing, right? Trans fats, including both industrial and natural trans fats, inhibit elongation of fatty acids by D6D... Does that make LA, ALA, and i-trans fats good? Maybe good for something but probably not good, IMO.
First, you can't take advantage of a slightly lower rate of biological aging if you're already dead of heart disease, so this would be a bad idea anyway

. But the main thing is direct displacement, not inhibitioin of
desaturation (not, NB,
elongation) by D6D -- although mutual competition for each step in the desaturation and elongation pathways separating the EFA per se from the long-chain derivative of the same series is an additional benefit to taking in the short-chain, essential PUFAs (ALA and LA) instead of the long-chain products (EPA, DHA, AA), which overrride metabolism and 'dump' the long-chain products directly into the membranes.
References1. Brochot A, Guinot M, Auchere D, Macaire JP, Weill P, Grynberg A, Rousseau-Ralliard D.
Effects of alpha-linolenic acid vs. docosahexaenoic acid supply on the distribution of fatty acids among the rat cardiac subcellular membranes after a short- or long-term dietary exposure.
Nutr Metab (Lond). 2009 Mar 25;6(1):14. PubMed PMID: 19320987; PubMed Central PMCID: PMC2670308.
2. Soriguer FJ, Tinahones FJ, Monzón A, Pareja A, Rojo-Martínez G, Moreno F, Esteva I, Gómez-Zumaquero JM.
Varying incorporation of fatty acids into phospholipids from muscle, adipose and pancreatic exocrine tissues and thymocytes in adult rats fed with diets rich in different fatty acids.
Eur J Epidemiol. 2000 Jun;16(6):585-94. PubMed PMID: 11049103.
Edited by Michael, 28 May 2010 - 05:39 PM.