I don't think that rodents are a particular relevant model for human aging when it comes to the impact of fatty acid metabolism. In contrast to mice, we are evolutionary adapted to consume significant amounts of HUFAs
Whether or not we're evolutionarily adapted to consume significant amounts of HUFAs (and I'm inclined to accept that we are), you have to remember that we're evolutionarily adapted to "nasty, short, brutish" lives, where the threats to survival are tigers, freezing, tuberculosis, and war, and in which intense daylong physical activity mitigates a range of harmful effects. The fact that we evolved under certain circumstances and may have been selected against or even coevolved with them doesn't mean they help us as regards longevity.
In any case, there is significant data that the relationships involved also apply in humans. Despite what you would assume from our regular evolutionary consumption of HUFA, we actually have exceptionally low levels of DHA and other HUFA in our tissue membranes. Indeed, our skeletal and liver membrane peroxidizability indexes are far lower than other mammals of the same size, or that would be predicted from the general relationship between body mass and PI:
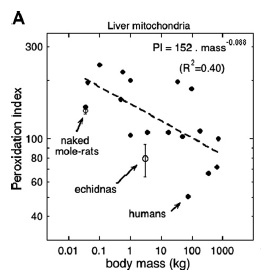
The relationship between the peroxidation index of liver mitochondrial phospholipids and body mass (A) of mammals.(2)
... and the membrane peroxidizability index of humans and other organisms that are exceptionally long-lived for our body size have maximum lifespans in the same relationship as all other mammalian species:
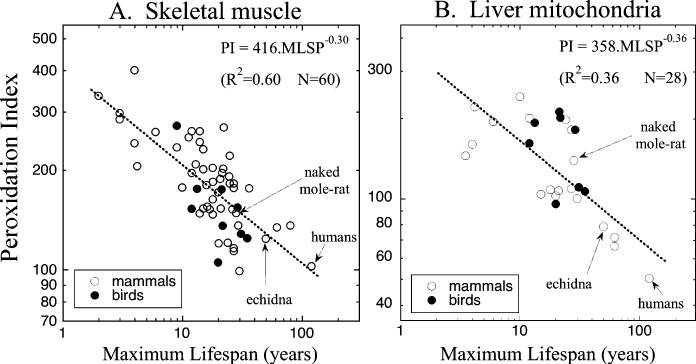
Skeletal muscle data combined from Valencak and Ruf (2007) and those cited in Hulbert (2005); liver mitochondrial data combined from Pamplona et al. (1998) and those cited in Hulbert (2005). Data for naked mole-rat from Hulbert et al. (2006b); echidna from A. Hulbert (1) Whole figure from (2).
... and there is some preliminary evidence that low PIs are linked to exceptional longevity in individual humans and genetically long-lived families:
Measurement of the fatty acid composition of human erythrocyte membrane lipids, as well as in vitro measurements on erythrocytes, has shown that centenarians have a reduced susceptibility to peroxidative membrane damage (Rabini et al. 2002). Part of longevity variation is genetic in origin. Studies of Danish twins suggests the heritability of longevity is 0.23 for females and 0.26 for males (Herskind et al. 1996); hence children of centenarians have been used as a model system for the study of the genetic basis of human aging (e.g. Atzmon et al. 2006). The fatty acid profile of erythrocyte membranes has been proposed as a potential biomarker of human longevity. A recent Italian study reported that the children of nonagenarians had erythrocyte membrane lipids with a PI of 64 (Fig. 6), which was significantly lower both than the value of 85 measured for a group of matched controls and the value of 83 for unmatched controls (Puca et al. 2008).[2]
(And note that erythrocytes are actually the weakest case to look at, based on the animal evidence: you would expect a stronger link in postmitotic tissues like skeletal muscle).
- and we have a much larger brain to supply with them in proportion to our body weight.
Yes, but we also eat a lot more food  . In the animal feeding studies, distribution of HUFA is differential in different tissues, based on their metabolic requirements, and DHA and EPA in brain are as a result far less susceptible to dietary manipulation than other tissues (see studies in original post). And there is no evidence of neurological disorders or increased risk of age-related cognitive declines in veg(etari)ans generally: indeed, there is (very limited) evidence of protection against cognitive decline and dementia in veg(etari)ans,(3-5) even though most veg(etari)ans really should be getting more omega-3 than they do (and of course get little to no HUFA, except for the extremely rare and recent fraction of them taking high-DHA algae supplements or DHA-fortified eggs, which are trends to recent to be impacting the epidemiological evidence) and also despite the confounding fact that B12 deficiency is shockingly common in vegetarians and especially vegans.
. In the animal feeding studies, distribution of HUFA is differential in different tissues, based on their metabolic requirements, and DHA and EPA in brain are as a result far less susceptible to dietary manipulation than other tissues (see studies in original post). And there is no evidence of neurological disorders or increased risk of age-related cognitive declines in veg(etari)ans generally: indeed, there is (very limited) evidence of protection against cognitive decline and dementia in veg(etari)ans,(3-5) even though most veg(etari)ans really should be getting more omega-3 than they do (and of course get little to no HUFA, except for the extremely rare and recent fraction of them taking high-DHA algae supplements or DHA-fortified eggs, which are trends to recent to be impacting the epidemiological evidence) and also despite the confounding fact that B12 deficiency is shockingly common in vegetarians and especially vegans.
Moreover, the literature is full of examples where rodent studies have shown toxicity from fatty acids completely benign to humans - a notorious example is erucic acid, which has long been considered toxic (and was therefore bred out of rape cultivars used for the production of canola oil) based on rodent studies, but has in fact traditionally been consumed in significant amounts in parts of Europe and India and never shown any toxicity. Studies with pigs have shown no toxicity as well.
Well, there is toxicity in nursling pigs, at least.(6) But IAC, (a) we're not talking about toxicity per se, and (b) part of the background driving the Hypothesis is the strong relationship between membrane HUFA and lifespan across multiple species and across multiple taxa.
Anyway, the basic hypothesis "highly unsaturated = highly unstable = increased oxidation = accelerated aging" seems too simplistic to me to really take it seriously.
I'm not at all clear what makes you think this is simplistic. IAC, there's no sense having an allergy to simplicity  .
.
Anyway, the basic hypothesis "highly unsaturated = highly unstable = increased oxidation = accelerated aging" seems too simplistic to me to really take it seriously.
And this is still very polite! (As you always are, other than me...) All the evidence we have shows the exact opposite.
Er, to what evidence would you point as showing the exact opposite?
I will also remind all that the dietary implications here are primarily and perhaps exclusively for people on CR: the rest of the population has the species-specific resistance to membrane HUFA incorporation, but doesn't have the additional HUFA-suppressing background metabolism observed in CR animals that would be expected to be disrupted by HUFA in the diet.
References
1: Hulbert AJ. Explaining longevity of different animals: is membrane fatty acid composition the missing link? Age (Dordr). 2008 Sep;30(2-3):89-97. doi: 10.1007/s11357-008-9055-2. Epub 2008 May 31. PubMed PMID: 19424859; PubMed Central PMCID: PMC2527634.
2: Hulbert AJ, Beard LA, Grigg GC. The exceptional longevity of an egg-laying mammal, the short-beaked echidna (Tachyglossus aculeatus) is associated with peroxidation-resistant membrane composition. Exp Gerontol. 2008 Aug;43(8):729-33. doi: 10.1016/j.exger.2008.05.015. Epub 2008 Jun 11. PubMed PMID: 18586080.
3: Sanders TA. Plant compared with marine n-3 fatty acid effects on cardiovascular risk factors and outcomes: what is the verdict? Am J Clin Nutr. 2014 Jun 4;100(Supplement 1):453S-458S. [Epub ahead of print] PubMed PMID: 24898234.
4: Sanders TA. DHA status of vegetarians. Prostaglandins Leukot Essent Fatty Acids. 2009 Aug-Sep;81(2-3):137-41. doi: 10.1016/j.plefa.2009.05.013. Epub 2009 Jun 4. Review. PubMed PMID: 19500961.
5: Giem P, Beeson WL, Fraser GE. The incidence of dementia and intake of animal products: preliminary findings from the Adventist Health Study. Neuroepidemiology. 1993;12(1):28-36. PubMed PMID: 8327020.
6: Food Standards Australia New Zealand (June 2003) Erucic acid in food: A Toxicological Review and Risk Assessment Technical report series No. 21; Page 4 paragraph 1; ISBN 0-642-34526-0, ISSN 1448-3017
































































