I have often thought that there should be a forum on longecity devoted to investing in biotechs. The collective knowledge of this board is fairly extensive and could be helpful in the biotech investing world! I myself am fairly clueless when is it comes to science compared to most here, however I have been investing in small cap biotechs for over a decade. Right now I am invested in AGEN, and believe that this year could prove to be a blockbuster year for it. Within the coming weeks, I would be looking to get in if I was new looking to invest in it. Here is a recent article ( The meat of Agen is not in their glioma heat shock vaccine, but rather their qs 21 adjuvant and the herpes vaccine.)
Agenus: Near-Term Drivers With A Market Opportunity Over $17 BillionApr 29 2013, 08:15 |
8 comments | about:
AGEN, includes:
ACAD,
CLDX,
GSK,
THLDDisclosure: I am long
AGEN.
(More...)Today, we will take a detailed look into a company that has an imminent catalyst, a number of important Phase II and Phase III data releases throughout 2013, and a product pipeline with potential to tap into a $17B market.
Agenus (AGEN) is a promising biotech company with two exciting platforms that focus on developing breakthrough immunotherapies for cancer and infectious diseases. Using their innovative Heat Shock Protein (HSP) and Saponin platforms, Agenus has developed a potentially lucrative pipeline of groundbreaking technologies and product candidates. Its products under development include QS-21 Stimulon adjuvant, which is in Phase III clinical trials for the treatment of malaria, melanoma, non-small cell lung cancer, and shingles, as well as for the treatment of various infectious diseases, multiple cancer types, and Alzheimer’s disease; and HerpV, a therapeutic vaccine candidate that is in Phase II clinical trial for the treatment of genital herpes.
Agenus' developmental pipeline focuses on therapies for some of the world's most disconcerting diseases. Their
QS-21 Stimulon Adjuvant aims to strengthen the body's immune response to a vaccine's antigen and is being tested on Malaria, Melanoma, Shingles, Alzheimer's Disease, Genital Herpes, and Non-Small Cell Lung Cancer. Additionally, their Prophage series provides therapies for Renal Cell Carcinoma, newly diagnosed Glioma, and also recurrent Glioma. Aligned with powerful partners like
GlaxoSmithKline (
GSK) and Janssen, Agenus is currently under speculated and I believe investors have yet to valuate the potential of their pipeline.
Agenus' Platform and Pipeline:
Heat shock proteins are a type of protein with the purpose of regulating the timing and conformation (shape) of cells throughout the body. They are "chaperones" for cells in the sense that they bind to the peptides in a cell and form a complex, so that our immune system can recognize diseased cells. When a diseased cell is no longer able to function and dies, heat shock proteins are released from the cell and our body uses as a signal to generate an immune response. To put it simply, Agenus takes advantage of this natural immune response by
utilizing heat shock proteins as vaccines against cancers and infectious disease.Agenus uses its heat shock protein platform for its Prophage Series of vaccines, which include Oncophage for Renal Cell Carminoma, G-100 for newly diagnosed Glioma, and G-200 for recurrent Glioma.
The Saponin Platform consists of the QS-21 adjuvant,
which is designed to strengthen the body's immune response to a vaccine's antigen, thus making it more effective. Saponin is actually extracted from the bark of the Quillaja saponaria tree and testing so far has shown that QS-21 is a safe adjuvant for a number of vaccine antigens.
According to the
company's website, when QS-21 is incorporated into vaccines it has been shown to:
- Demonstrate efficacy via parenteral or mucosal administration routes
- Potentiate both prophylactic and therapeutic vaccines
- Potentiate immune responses to viral, bacterial and parasitic antigens, including recombinant proteins, polysaccharide antigens, DNA vectors, conjugated antigens, multivalent vaccine formulations and notoriously weak antigens
- Exhibit a favorable safety profile
- Integrate easily with most excipients and formulations
- Synergize with other adjuvants as well as approaches using carrier proteins
From above, it is evident why a powerful pharmaceutical company such as GlaxoSmithKline is partnered with Agenus. QS-21 Stimulon Adjuvant can certainly help build market share for Glaxo's current drugs by increasing the efficacy of each. Thus, it is reasonable to speculate that other large pharmas could come knocking on the Agenus' door with partnering interest. Agenus already states that it has 10 other indications possible for this platform, so maybe they have been working on that already.
Some highlights from Agenus' QS-21 adjuvant include successful Phase III RTS, S: Malaria, Phase III MAGE A3: Stage IIIB/IIIC Melanoma, Phase III MAGE A3: Stage IB-111A NSCLC, and Phase III Herpes Zoster: Shingles. All of those indications are partnered with GSK. Additionally, AGEN is partnered with Janssen for Phase II ACC-001: Alzheimer's Disease.
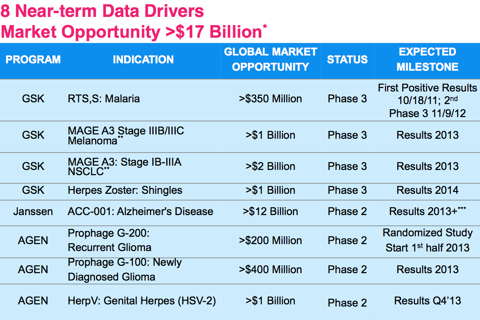
Together, the global market opportunity is over $17 billion for these products. The partnered products have the potential to bring Agenus over $100M a year royalties. The final 3 products on the chart are un partnered. If Agenus management executes its long term goals correctly, these 3 products can potentially bring the company over $1B in revenues. With a current market cap under $120 million, AGEN seems to be undervalued.
From the company's
earnings call on April 24,
Garo Armen, Chairman and CEO of Agenus, remarked:
It is (QS-21) currently being studied in 17 clinical programs in development. Let me now switch gears and discuss the status of our internal development programs. As I mentioned earlier, our HerpV randomized double blind, multi-cancer Phase 2 trial in individuals infected with HerpV 2 is fully screened for enrolment. This puts us on track to report initial results during the fourth quarter of this year. I would like to note that HerpV is the most advanced therapeutic vaccine in clinical development today. This study is evaluating the efficacy of HerpV vaccine by measuring viral shedding before and after vaccination. In the study 65 participants will receive HerpV which contains QS-21 and a control group of 10 participants who will receive placebo. A booster injection will be given at six months after the initial treatment to evaluate both the potential of delayed effect as well as the durability of treatment effect. Key opinion leaders in the field have helped design the HerpV Phase II study and HSV-2 experts believe that a reduction in viral shedding, the driving force behind the spread of genital herpes is a key surrogate which could be predictive of clinical benefit defined by reduction of disease outbreaks. Among the unique attributes of HerpV is the fact that it contains 32 HSV-2 derived immunogenic antigens and was designed with the intent of treating a broad population of HSV-2 infected individuals. We believe that if HerpV is ultimately shown to be a safe and effective product in late stage trials, it would represent a breakthrough in the treatment of herpes.






















































