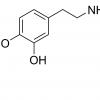
If somebody has a legitimate source for NSI-189, please pm me. Seems to have gotten scare since their lawyers gave ceretropic a warning!
That actually happend? Seriously, that was only a matter of time. I really wondered what all those individuals who try to make a buck with a patented molecule have in their minds. They can be lucky they got a warning, something like that could have ruined their enterprise completely.
I hope neuralstem will pubilish the dose-response curve of nsi, I'd love to know what is the most effective dosage is.
This is part of the slide presentation. The most interesting finding is that NSI-189, in a albeit small study, has better MARDS scores then any other antidepressant on the market. (http://www.nejm.org/...=articleResults) See summary appendix and figure 3.
1) SDQ data looks pretty darn good! Results were statistically significant at Day 28 (p=0.02) and Day 84 (p=0.03). No real dose response, suggesting that 40 mg QD may offer target saturation. Effect size (Cohen's d) was 0.9 at Day 28 and 1.1 at Day 84 (suggestive of large effect).

Source: ASCP - M. Freeman, 2014
2) MADRS data looked just ok. Not quite as strong as SDQ, but separation from the placebo (albeit not statistically significant). Higher doses of NSI-189 looked to produce a more sustained effect, but p-values for individual cohorts was not provided.

Source: ASCP - M. Freeman, 2014
3) CGI-I data was inconsistent, although did show a trend toward improvement by Day 84, especially with the highest dose of 40 mg TID (yellow line). We suspect that the 40 mg TID dose did offer statistically significant separation from the placebo, although this was not reported and the trial was not large enough to report individual cohort statistics.

Source: ASCP - M. Freeman, 2014
4) CPFQ data was the most impressive. This is Massachusetts General Hospitals own scale to assess depression and physical function (note the study took place at MGH). Results look excellent, with clear separation from the placebo at both Day 28 and Day 84 (both p<0.01) and strong effect size. Higher doses of NSI-189 looked slightly better.

Source: ASCP - M. Freeman, 2014
More Data Next Week
Next week, at the 29th CINP World Congress of Neuropsychopharmacology meeting, additional data will be presented on the effects of the drug. Data should include quantitative electroencephalography (qEEG) discussion. We reached out to management to ask about MRI data. We were told that MRI data probably would not be presented at CINP, but that his analysis was still ongoing. So perhaps we will see MRI data when the Phase 1b results are published in a peer-review journal - which we fully expect to take place.
An
abstract of the data can be found on the CINP website. We present that abstract below:

Source: CINP Website, 2014
Summary Thoughts
We caution investors that these are very early-stage data in only 18 patients on active drug. The study was designed to assess safety and tolerability and not powered for efficacy endpoints. As such, reading too much into the results (bullish or bearish) is imprudent. However, in terms of a Phase 1b study, this looks very good. Safety was confirmed and there are clear trends toward efficacy. Next step is a multicenter Phase 2 study more statistically powered to show separation on the efficacy endpoints over a longer duration of time. We will have additional analysis after the presentation at CINP.
http://bionapcfa.blo...-ascp-oral.html