Human and Mouse Life Extension DIYBio Stem Cells Experiment
This project idea is rather a suggestion than a proposal, for what - I think - we, the ImmInst/Longecity and also the global life extensionist / immortalist community, should do and bring into effect / realize.
This project has multiple parts: a science & research, an outreach campaign and a financial/business one.
Also this is an initial outline, for consideration, discussion and expansion, modification, etc., as I imagine the whole thing as a community project, that is the members of the community do the further planning and refinements, etc, then a couple of people do this or that part of it.
Some background on this project article:
I have had some vague ideas for a project like this already for a while now, and then I've found some similar ideas here in the forum, and I considered that a project based on these may be a good idea, so I decided to write them down and design a project framework. So this is based on my and some of those similar ideas of others from the forum:
the various related topics' list:
t1) Project Ideas/ Do-It-Yourself Biotech
t2) Bioscience/ Young Blood Reverses Signs of Aging in Old Mice
t3) Bioscience/ Removing Senescent Cells Halts Many Signs of Aging
t4) Project Ideas/ More Knowledge About Removing Senescent Cells
t5) SENS & Methuselah/ MFURI Team Meeting Notes
t6) BioscienceNews/ Old Blood Versus Young Blood From a Programmed Aging Perspective
t7) Bioscience/ Blueprint for biological immortality
and possibly other ones.
In the text below I will give the place of the forum where the relevant similar idea was mentioned, with the above topic numbers / the number of the post, like: [t1/1].
Leaders: see below
Funding required: yes.
Funding level: variable, see below.
Science / Research
The science part is the research, and it would be a multiple step experiment (Step 1-6), each of them is useful alone in itself, and for the whole research as well. Its approach would be through the extracellular environment in the direction: from the exterior to the interior parts (see Figure 1), that is from: blood, interstitial/tissue fluid, cellular microenvironment, stem cell niche.
Because increasing number of evidences suggest that:
- even the old cells and tissues still have good regenerative ability, just their aged environment inhibits it [e.g. Sci5, Sci2], and
- the matter named as cellular senescence and aging is partially the effect of the old and unhealthy cellular environment, and that
- the young environment has regenerative / rejuvenation capability on the cells [e.g. Sci1, Sci4].
For more about the science background see below at the Scientific literature. Also the [Sci#]s refers to that list.
The aim of the project and experiments would be to measure the exact levels of these abilities and effects, on the short, mid- and long term, and to test their possibly life extension capacity as well, and also the possible adverse effects on the mid- and long run (for some discussion see [t6/1,4]).
An illustration from [Sci7], Figure 1:

First there is a general summary list, and then some details and illustrations.
Note: all of these are based on techniques that are already established more or less, with some modifications. And the starting two steps are the easier, simpler and cheaper ones, and then ther is an increasing complexity towards the later ones.
The steps:
1) mice young blood and only plasma/serum to old recipient injections (and possibly vica versa)
[t2/3, t4/11, t6/1]
note: for humans see the Outreach part below.
2) antibody treatment against the pro-aging molecules in the old blood
[t3/96)
3) in vitro human and mouse old and young cell culturing on young and old blood serum medium, respectively, and with stem cells provided environments; that is co-culturing, like in [Sci2], with the goal to create an ideal environment, like medium, etc. for optimization of the cell culturing to achieve and maintain the good health and proliferation capacity of the cells. Like reducing the oxidative stress, defending the cell and DNA integrity and the telomere lengths, etc.. One example: [Sci6].
This step is based on another current research study of mine: called 'Beyond the Hayflick limit', with the sub-title of 'Cell immortalization without transformation', that is reaching that state without the malignant genetic alterations. Initially and preferably with optimization on the extracellular level, or, if needed, with some intracellular intervention, like: gene expression and/or positive genetic modifications, i.e. gene therapy.
4) artificial autologous young blood creation, with ex vivo/in vitro stem cell based cell cultures and tissue-engineering, i.e. generation of required tissue layers, in bioreactors, like hematopoietic/blood stem cells based red bone marrow and separate liver tissue, etc., maintained with continuous repair in a constant young state, based on Step 3, without the aging related changes.
Also design and create a machine that produces this automatically.
Then with this bio-artificial blood and plasma/serum repeating the 1st and 3rd steps.
[t2/7]
That is replacing periodically the old blood with the young one.
The point of this step is to try to maintain an as much as possible optimal, constant young and healthy in vivo environment, which was determined with the help of Step 3, for all of the cells and tissues of the body, through the manipulation of the blood, that is an indirect intervention into the interstitial fluid in the extracellular space. I.e. this route would be the therapeutic pathway. Or with a direct one later with microneedles and such.
This step may also include an immune system repairment, and a blood purifier [t2/10], similar to a dialysis machine, to filter out / destroy the pathogens, and thus minimalize their harmful effects and lessening the immune system's need to engage them in possibly tissue destructing wars.
5) in a scale related to the percentage of regeneration/rejuvenation achieved in the above methods, repeating the 4th step with all other tissues and organs, with organ specific progenitor and generally with induced pluripotent stem cells.
Then periodically monitoring the body and, if necessary, repairing the age-related damages in the tissues and organs with pre-checked healthy stem and differentiated young cell/tissue injections/implants, i.e. 'whole body regeneration'. [t1/7, t7/1] This can be considered a variant of the cell / stem cell therapy.
And this step also includes the development of automatized machine systems which create these, and possibly bioprinting of tissues.
The point here would be that a minor copy for all of the body's important organs' tissues are created and maintained/stored ex-vivo to be available in a young state.
These 3-5 steps also include a checking procedure at the cellular / tissue and the intracellular and nucleus level, namely e.g. of the integrity of the DNA and chromatin structures, before and after the regeneration, both in the body's and cultures' cells.
6) refinement of these methods for humans, for creating a prototype of an automatic system for a clinical setting for the trials and therapy, and if it's good, then repeating the 4th-5th steps indefinitely.
The funds needed: for Step 1-2: about 1000$ per step;
the costs for Step 3 and onwards are greater and more highly variable, but about 5000-10000$ per step or around 15000-25000$ in total; however these are just preliminary estimates, because the total sum depends on a number of factors. Also important that this would be in a DIYBio [t1/16] methodology for the reduction / minimalization of the costs. So these later steps need further examinations and planning / designing. Also worth noting that about 3/4 of the costs are the laboratory equipment, which represent value and remain usable afterwards.
Leaders: for the project leader, especially for Step 1-2, I'd suggest AgeVivo, since he is probably the only person in this forum, at least that I know of, who is capable to carry out the experiments with the mice (and regarding Step 1, the similar ideas were in his posts, after all), and also dedicated enough to do it; possibly as an expansion to his already running MPrize@home [t5/4] project.
Either together with the cell culture lab or this one in a separate location with other persons.
If he or they is/are willing of course; if not, then we will have to search and find other persons and locations to conduct the research experiments. In either case it will be, if needed, with 1 or 2 recruited assistants for a few hours per week, either volunteers or paid people.
I, Avatar of Horus, would be a scientific advisor, researcher, and designer, constructor, engineer, bioinformatician, organizer, overseer, etc. of the project.
Some details and illustrations:
Step 1:
For illustration see the pictures below.
This step needs only the mice, cages and some needles and syranges, tubes and a lab centrifuge, such a machine can be purchased for around 400-500$.
The exact number of the animals is to be decided, but possibly something like a minimum of 6-8 animals in two lots, that is two parallel experiments with 3-4 old and young mice in each. Possibly without controls, the controls would be all the untreated mice of the present and past.
In the time scale: this would produce measurable results almost immediately from the beginning, like in a week, and then countinuously after that in every weeks and months. The total time that it would take cannot be said currently - but hopefully the animals would be long lived - since actually this is the aim of the experiment, i.e. the time is what's needed to be known and found out.
The measurements would be with numbers, 1-6, on two related scales: one for the age of the animal, like: 'juvenile, youth, adult, middle aged, old, very old'; and the second is the age-matching scale in the state of the animal like: 'vigorous, ..., to vegetative'.
Here also the quantity of the blood and periods between the injections need to be determied.
The illustrations:
the procedure, created from and based on [Sci4 / Figure 2a]:

the blood [from the Internet]:

the blood fractionation and the plasma/serum:


a real vacutainer, from Wikipedia:

Step 2
The antibody treatment's specifics: this is based on [Sci4 and its Figure 4], and it would be antibody injections against a pro-aging protein in the blood with 'rat IgG2a neutralizing antibody against mouse CCL11 (R&D Systems, Clone: 42285)', it costs $329 / 500 ug, and that's enough for around 50-100 doses.
More info: Mouse CCL11/Eotaxin Antibody http://www.rndsystem...20/Applications
This step consists of another lot of mice and the results would be compared to those of the Step 1.
Step 3
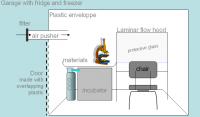
fume hood / biosafety cabinet for cell culturing work [from the Internet]:

Some graphs of human and mice cell cultures that show the relevancy of this step:
The point is the young and old cells' number and regeneration in on old and young serum based medium and with stem cells.
from [Sci2/ Fig.1]:

from [Sci2/ Fig.2]:

from [Sci2/ Fig.4]:

from [Sci2/ Fig.6]:

from [Sci3/ Suppl. Fig.5]:

from [Sci3 /Suppl.Table 1]:

from [Sci4/ Suppl. Fig.13]:

effects of the increased level of oxidative stress:
growth: from [Sci6/Fig.1A]

chromosomal abnormalities: from [Sci6/Fig.2B]

cell senescence and telomerase activity::from [Sci6/Fig.3AB]

In this step the exact levels of the enhanced proliferation and regeneration and integrity defense abilities in the optimized environment need to be measured and also checking these methods with other cell types.
The results will be seen in days or about a week form the start and continuously after that.
Step 4
tissue culture flasks [from the Internet]:

a bioreactor [from the Internet]:

some of the bio-artificial blood's cells and their creation:

Step 5
stem cell therapy

For the checking: e.g. microscopy, and AI based computer vision system that can identify the defective cells, and other methods.
e.g. pictures of chromosome stability/in-: from [Sci6/Suppl. Fig. 1]:

another one [from the Internet]:

The further details of the 3-6 steps are to be described later if they become actual and necessary.
Outreach campaign
First a reaching out will be needed to those people who may be interested in contribution and support for this sort of experiment project.
Also I see some possibilities for other outreach campaigns. These would be not only for the general public, but initially with a focus on the medical establishment / hospitals, especially on the blood processing / transfusion medicine parts of that, first with the goal to get data from them regarding the human blood and plasma/serum transfusions of the past, since these could be considered as partial human clinical trials for the Step 1 above, which may be examined from the young/old perspective. Then because the mentioned evidences also suggest that the old blood may have negative health effects on the younger recipients. And therefore this should be drawn to the doctors' and patients' attention and tested more extensively than in this little experiment. And considering the possible importance and significance of this, the general public too may be interested; and for these reasons this campaign may hold the promise of even a larger effect. These would apply, of course, to the possibly positive, the regenerative and life extension effects as well.
For example a related mainstream media coverage of [Sci4] in an article: Young blood reverses effects ageing http://www.guardian....-effects-ageing
Also there is another possibility for establishing connections to other bio/med students and experts and universities, labs, etc., and initiating research collaborations with them.
Also of course a campaign to the finance circles, but this is connected and leads to the following part, the Business, so see there.
In these campaigns to these groups hopefully there will be some people who may join our efforts.
Business
This part [t2/22, also more generally the topics below] covers the possible products on the mid/long term, like: the autologous bio-artificial blood, and regenerative/anti-aging 'young blood supplement' [t2/12], like a new VIMMORTAL, and a cognitive enhancer nootropic [t2/28], and the therapies, and also the machines, procedures and protocols. And since any degree of results in the experiments can possibly have financial consequences, I think a business part would be useful too, also for the reason that with this part even such people may be involved, who are interested only in the financials and not in life extension.
And this would be a biotech startup company, began with crowdfunding, with the writing of a detailed business plan and presentation and finding angel and venture investors, then building a top notch lab and factory, possibly making a stock market IPO, and establishing regeneration / rejuvenation / life extension clinics.
Illustration [from Wikipedia]:
The garage where two guys: Hewlett & Packard began their company

The somewhat related general business topics:
Bioscience/ Basement Biotech Startups
http://www.longecity...otech-startups/
Townhall/ ImmInst Goal Companies?
http://www.longecity...goal-companies/
The business idea(please do read :-) )
http://www.longecity...please-do-read/
Townhall/ Too much talk and too little action
http://www.longecity...-little-action/
Scientific literature
Some of the relevant literature:
1) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Conboy et al. 2005. Nature http://www.ncbi.nlm....pubmed/15716955
2) Loss of stem cell regenerative capacity within aged niches. ME Carlson and IM Conboy, Aging Cell(2007) 6. http://www.ncbi.nlm....pubmed/17381551
3) Molecular aging and rejuvenation of human muscle stem cells. Carlson ME et al. 2009. EMBO Molecular Medicine http://www.ncbi.nlm....pubmed/20049743
4) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Villeda et al., 2011, Nature http://www.ncbi.nlm....pubmed/21886162
5) Muscle transplantation between young and old rats: age of host determines recovery, Carlson BM and Faulkner JA, Am J Physiol. 1989 http://www.ncbi.nlm..../pubmed/2735398
6) Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. TS Li et al. Cardiovasc Res (2011) 89(1). http://www.ncbi.nlm....pubmed/20675298
7) Stem cells, ageing and the quest for immortality, TA Rando, Nature, Vol 441, 29 June 2006 http://www.nature.co...ature04958.html
and other related articles, like:
Historical claims and current interpretations of replicative aging. Wright WE, Shay JW., Nat Biotechnol. 2002 Jul;20(7):682-8. http://www.ncbi.nlm....pubmed/12089552
Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. RD Ramirez et al., Genes Dev. 2001 Feb 15;15(4) http://www.ncbi.nlm....pubmed/11230148
Aging of Hematopoietic Stem Cells is Regulated by the Stem Cell Niche. W. Wagner et al., Exp. Geron. 2008. http://www.ncbi.nlm....pubmed/18504082
etc., i.e. further similar studies.
So, if we decide to start and do any of these above - as it is also possible to do the parts and steps in
separate subsequent projects - I imagine the whole as performed in a reward credit system (similarly to the the forum's 'Thank you points'), that is everyone who participates and contributes in any way during the course of the project, in the case of success, would be rewarded accordingly in that startup company with a proportionate share.
Edited by Avatar of Horus, 11 April 2013 - 01:24 PM.