http://www.ncbi.nlm....pubmed/21557387
I'm not sure if it's a good study, but there's more
http://www.jneurosci...29/23/7619.full
Small world network architecture is very important for a properly functioning brain
Lostfalco, because ampakines wouldn't really help the PFC, could we use some "neurotoxins", i.e. NDMA agonists in low doses? We could use memantine to block excitotoxicity. How important do you think GABA is to cognition? Is it there mainly to quench anxiety or for calculations/computations? I will watch the video.
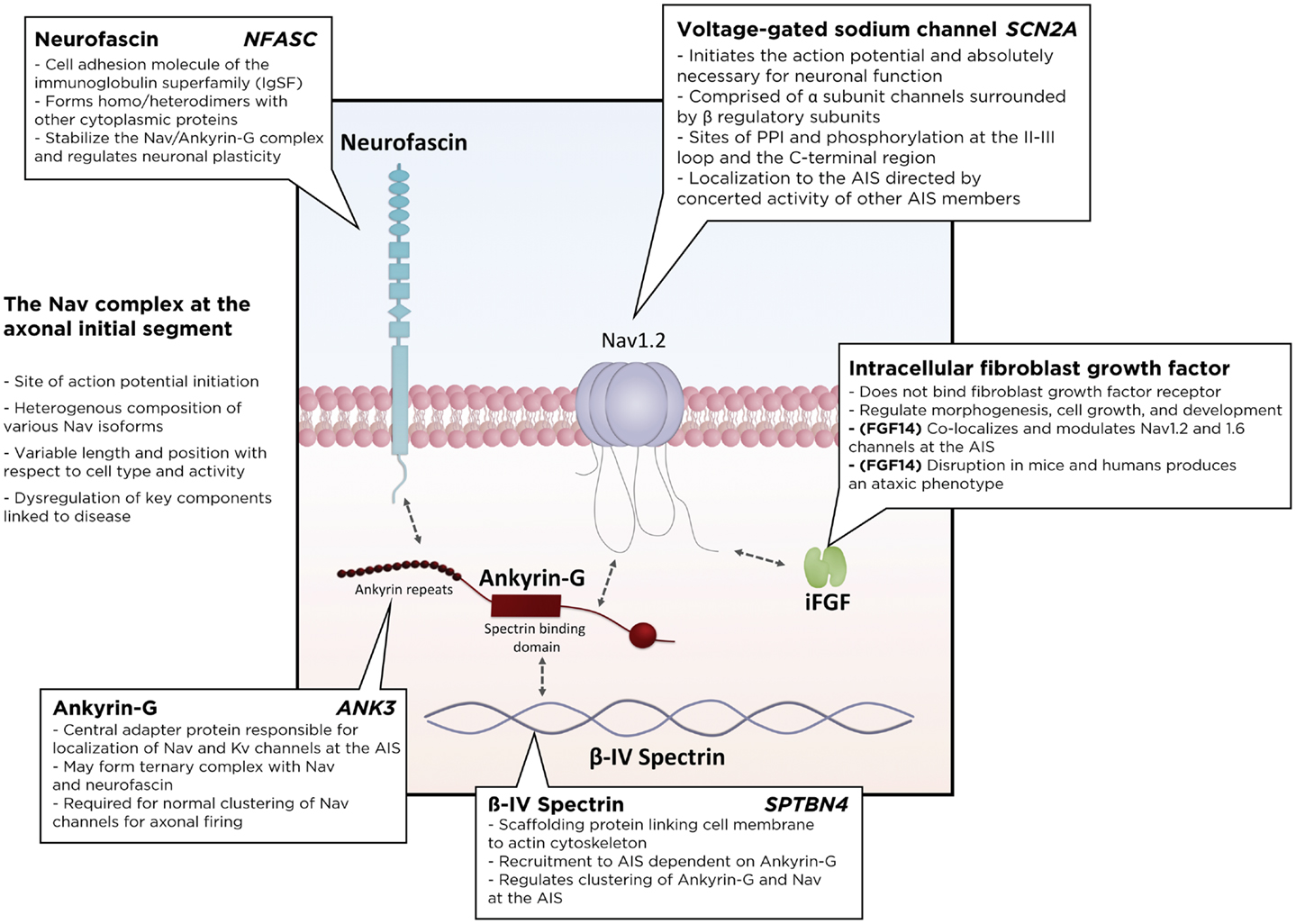
Sodium channels are incredibly interesting. Also, from what I've seen of the video, balance is key. Too much/little of any neurotransmitter will create disorder. How will we be able to tell what levels are optimal? Learning that Nicotinic a7 receptors displace the Mg is super cool.
You should look into polygala tenuifolia, it creates NR2B in the hippocampus and PFC if I remember correctly, I will look it up but it's hard to find.
Very nice finds, bigyellowlemon. Your thinking is right along the same lines as mine. I'm trying to increase both the speed of transmission AND the number of networks involved in order to create supranormal human cognition. Your articles both touched on the importance of efficient transmission caused by short range connections. This is partly why my focus is on synaptogenesis (insulin, igf-1, dihexa, 7, 8 dihydroxyflavone, souvenaid, magnesium l-threonate, etc. all contribute to synaptogenesis).
My thinking is this: 1. If I can create more synapses, then I can increase the short range connectivity of my neurons and then the excess synapses will later be pruned to increase efficiency when they are not used as much as the short range connections (hopefully). Does that make sense? Your articles mentioned that raw numbers of synapses were not predictive of intelligence which makes sense to me. Shorter connections would spur speed of transmission as would...
2. If I can increase the speed/probability of transmission between neurons/networks then I can increase connectivity and hence intelligence. I''m attempting to do this in the dlfpc with A7, NMDA, sodium channels, potassium channel blockage, cAMP reduction etc. Just some thoughts that I've been milling about. Thanks for the interesting studies! I'll have to pore over them in more detail when I have some time.
I think ampakines can contribute to overall network connectivity because of their involvement in regions other than the dlpfc. I still like IDRA-21 as part of this stack.
I'm attempting to avoid nmda blockers at the moment. If we keep our doses fairly low and our mitos functioning well, I don't think we have to be extremely concerned with excitotoxicity (we should be a little concerned though...never hurts to be safe). Our main concern is that we don't want too much calcium to stay too long in the cell...that's what causes interaction with mitochondria and apoptosis. We actually want our nmda receptors free of blockade and able to open in order to cause action potential transmission...as always, it's a bit of balancing act but I don't think we are at serious risk if we focus on low dosing at multiple, simultaneous targets.
GABA is very important for shaping and refining the signal so I wouldn't do anything to block it but it can also slow things down in excess. I think a slight tip in favor of glutamate over gaba should be sufficient for our purposes.
Optimal levels are super important as you mentioned and balance is key. I start with very low doses and work my way upward while keeping an eye on everything else. She talks about this in the video. A little bit of dopamine is good but too much causes chaos. The exact same thing is true of acetylcholine. This is why megadosing is for megafools. =)
Totally agree about A7 removing the mg block!
I'm familiar with polygala...I'll have to check which regions it works in as well.
Speaking of sodium channels...this study isn't in the prefrontal cortex but I'm gonna have to research this further to see if ghrelin works the same way there as it does in the pituitary. (MK-677 is a readily available ghrelin mimetic and ghrp-2 and ghrp-6 can be taken intranasally)
http://www.ncbi.nlm....pubmed/25151402
Endocrine. 2015 Apr;48(3):929-36. doi: 10.1007/s12020-014-0392-x. Epub 2014 Aug 24.
Ghrelin increases growth hormone production and functional expression of NaV1.1 and Na V1.2 channels in pituitary somatotropes.
Abstract
A variety of ion channels are expressed in the plasma membrane of somatotropes within the anterior pituitary gland. Modification of these channels is linked to intracellular Ca2+ levels and therefore to hormone secretion. Previous investigations have shown that the gut-derived orexigenic peptide hormone ghrelin and synthetic GH-releasing peptides (GHRPs) stimulate release of growth hormone (GH) and increase the number of functional voltage-gated Ca2+ and Na+ channels in the membrane of clonal GC somatotropes. Here, we reveal that chronic treatment with ghrelin and its synthetic analog GHRP-6 also increases GH release from bovine pituitary somatotropes in culture, and that this action is associated with a significant increase in Na+ macroscopic current. Consistent with this, Na+ current blockade with tetrodotoxin (TTX) abolished the ghrelin- and GHRP-6-induced increase in GH release. Furthermore, semi-quantitative and real-time RT-PCR analysis revealed an upregulation in the transcript levels of GH, as well as of NaV1.1 and NaV1.2, two isoforms of TTX-sensitive Na+ channels expressed in somatotropes, after treatment with ghrelin or GHRP-6. These findings improve our knowledge on (i) the cellular mechanisms involved in the control of GH secretion, (ii) the molecular diversity of Na+ channels in pituitary somatotropes, and (iii) the regulation of GH and Na+ channel gene expression by ghrelin and GHRPs.
http://www.ncbi.nlm....pubmed/19223651
Am J Physiol Endocrinol Metab. 2009 May;296(5):E1148-56. doi: 10.1152/ajpendo.90954.2008. Epub 2009 Feb 17.
Upregulation of voltage-gated Na+ channels by long-term activation of the ghrelin-growth hormone secretagogue receptor in clonal GC somatotropes.
Author information
- 1Laboratorio de Neuroendocrinología, Instituto de Fisiología, San Manuel, Puebla, México.
Abstract
A central question in adenohypophyseal cell physiology concerns the role of transmembrane ionic fluxes in the initiation of the hormone secretion process. In the current report, we investigated the effects of the growth hormone (GH) secretagogues ghrelin and GH-releasing peptide-6 (GHRP-6) on the regulation of the functional expression of voltage-gated Na(+) channels using the tumoral somatotrope GC cell line as a model. Cells were cultured under control conditions or in presence of the GH secretagogues (GHS) for 96 h, and Na(+) currents (I(Na)) were characterized in whole cell patch-clamp experiments. GHS treatment significantly increased I(Na) density in a dose-dependent manner. The effects of GHRP-6 were accompanied by an augment in conductance without changes in the kinetics and the voltage dependence of the currents, suggesting an increase in the number of channels in the cell membrane. Sustained inhibition of L-type Ca(2+) channel activity decreased I(Na) density and prevented the effects of the GHS, whereas long-term exposure to an L-channel agonist increased I(Na) density and enhanced the actions of GHRP-6, indicating that Ca(2+) entry through these channels plays a role in the regulation of Na(+) channel expression. Likewise, GHRP-6 failed to enhance Na(+) channel expression in the presence of membrane-permeable inhibitors of protein kinases A and C, as well as the Ca(2+)/calmodulin-dependent kinase II. Conversely, treatment with a cAMP analog or a protein kinase C activator enhanced both basal and GHS-induced secretion of GH measured by enzyme-linked immunoassay, suggesting that GHRP-6 acting through the ghrelin receptor and different signaling pathways enhances Na(+) channel membrane expression, which favors hormone release from GC somatotropes.
http://www.ncbi.nlm....pubmed/26401470
J Cachexia Sarcopenia Muscle. 2015 Sep;6(3):237-41. doi: 10.1002/jcsm.12028. Epub 2015 Apr 27.
One-year intranasal application of growth hormone releasing peptide-2 improves body weight and hypoglycemia in a severely emaciated anorexia nervosa patient.
Haruta I1,
Fuku Y1,
Kinoshita K1,
Yoneda K1,
Morinaga A1,
Amitani M1,
Amitani H1,
Asakawa A1,
Sugawara H2,
Takeda Y2,
Bowers CY3,
Inui A1.
Abstract
BACKGROUND:
In Japan, growth hormone releasing peptide-2 (GHRP-2) is clinically used as a diagnostic agent for growth hormone secretion deficiency, but the therapeutic application of GHRP-2 has not been studied in anorexia nervosa. GHRP-2 reportedly exhibits agonistic action for ghrelin receptor and increases food intake.
METHODS:
We administered GHRP-2 to a patient with a 20-year history of anorexia nervosa to determine whether GHRP-2 treatment increases food intake and body weight. GHRP-2 was administered before every meal by an intranasal approach for 1 year.
RESULTS:
Although the patient reported a decreased fear of eating and decreased desire to be thin by our previous treatment, she was unable to increase food intake or body weight because of digestive tract dysfunction. Vomiting after meals caused by delayed gastric emptying and incurable constipation were prolonged, and sub-ileus and hypoglycemia were observed. GHRP-2 increased the feeling of hunger and food intake, decreased early satiety and improved hypoglycemia. The patient's body weight gradually increased by 6.7 kg (from 21.1 kg to 27.8 kg) in 14 months after starting GHRP-2 administration. The fatigability and muscle strength improved, and the physical and mental activities were also increased. No obvious side effects were observed after long-term intranasal administration of GHRP-2.
CONCLUSIONS:
Patients with a long-term history of eating disorder occasionally recover from the psychological problems such as fear for obesity but remain emaciated. We believe that ghrelin agonists such as GHRP-2 may be promising agents for the effective treatments of severe anorexia nervosa in a chronic condition.
Edited by lostfalco, 20 January 2016 - 03:21 PM.