
TULIP Research Thread: Mitochondria/ATP/Biophysics, etc.
#31
Posted 04 October 2013 - 11:21 PM
#32
Posted 06 October 2013 - 12:01 AM
#33
Posted 09 October 2013 - 10:38 PM
#34
Posted 10 October 2013 - 12:17 PM
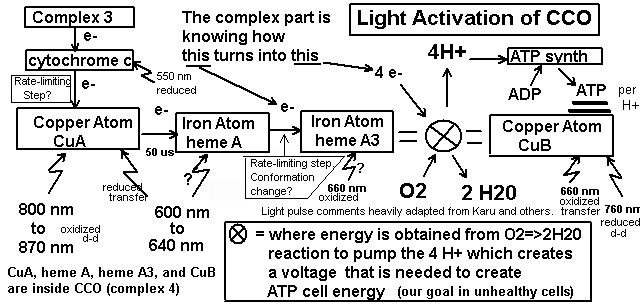
Below is an image of my interpretation of several papers that I read several years ago. If I spent a week trying again, maybe I could come up with a more useful pulse sequence than 50 us on, 250 us off. I can do easily do 1 us second pulses, so the time frames are not an electronics problem. A rudimentary knowledge of electronics allows me to cascade 555 timers and potentiometers for variability without having to figure out how to do a programmable microcontroller with digital display. "Bimetal core" is referring to where a copper and iron atom are splitting O2 and creating H2O by transferring electrons into this "fuel cell" reaction, while pushing H+ atoms to where they are needed.
.

Edited by zawy, 10 October 2013 - 12:32 PM.
#35
Posted 10 October 2013 - 01:49 PM
I think most of the discussion on 660 nm and 850 nm is mostly from my writings on this subject. 630 nm, 670 nm, and 880 nm used to be the emphasis by companies making products like anodyne and warp10 (nothing wrong with warp10 except cost). I've been trying to get elixa customers to get them to change to 850 nm from 880 nm for years. My insistence over the last 9 years that there is no difference in the quality from the difference could also be wrong: perhaps the 760 nm or 660 nm are forcing a completion of the O2 to 2H2O reaction before all 4 e-'s have come into the bimetal core. This could use up negatively-charge reactive oxygen species (like O2-)that are in the mitochondria allowing a less toxic a alkaline state. Or produce an H2O+ if such a thing exists, that would then use up toxic ROS. Similarly, 850 nm could do the same thing by prevent e- leakage out of the transport chain which causes ROS. So the comments I've seen that the benefit is from producing ROS is confusing because older papers and my reasoning say it should prevent it. However, if there is a build up of H+ when ATP is not being consumed by activity, then it could force more e-'s out of the transport chain that then cause ROS. So the combination of light therapy and exercise is a great idea, like our ancestors being out in the sun and working at the same time: not a coincidental situation, but evolutionarily instigated. So I am not sold on ROS theories. Certainly mild ROS from exercise helps. But I do not regard that as a reason to avoid light therapy and vitamin C before exercise. I think they should be used to help do a harder exercise until the right ROS is generated. Then do then again 2 hours later. Enough to stress the cells, but then give them the tools to repair themselves and repeat the next day, more frequently and/or harder than without the vitamin C and light. A guy called me once who was serious about using halogen lights and exercise at the same time. He wanted to build a treadmill with about 2,000 watts of halogen lights point at the runner. I encouraged him but emphasized he had to have water blocking and the people had to be naked on the muscle areas.
#36
Posted 10 October 2013 - 02:22 PM
#37
Posted 10 October 2013 - 05:10 PM
#38
Posted 10 October 2013 - 05:25 PM
Edited by zawy, 10 October 2013 - 05:38 PM.
#39
Posted 17 October 2013 - 07:19 PM
Abstract: http://www.ncbi.nlm....pubmed/24042162
Free Full-Text: http://jpet.aspetjou...113.208017.long
#40
Posted 17 October 2013 - 11:29 PM
http://www.ncbi.nlm....pubmed/23219040
Edited by lostfalco, 17 October 2013 - 11:30 PM.
#41
Posted 04 November 2013 - 11:52 PM
http://www.ncbi.nlm....les/PMC2957070/
#42
Posted 11 February 2014 - 09:04 PM
http://www.ncbi.nlm....pubmed/23946409
J Neurosci. 2013 Aug 14;33(33):13505-17. doi: 10.1523/JNEUROSCI.0918-13.2013.
Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer's disease.
Meng C, He Z, Xing D.
Author information
Abstract
Downregulation of brain-derived neurotrophic factor (BDNF) in the hippocampus occurs early in the progression of Alzheimer's disease (AD). Since BDNF plays a critical role in neuronal survival and dendrite growth, BDNF upregulation may contribute to rescue dendrite atrophy and cell loss in AD. Low-level laser therapy (LLLT) has been demonstrated to regulate neuronal function both in vitro and in vivo. In the present study, we found that LLLT rescued neurons loss and dendritic atrophy via upregulation of BDNF in both Aβ-treated hippocampal neurons and cultured APP/PS1 mouse hippocampal neurons. Photoactivation of transcription factor CRE-binding protein (CREB) increased both BDNF mRNA and protein expression, since knockdown CREB blocked the effects of LLLT. Furthermore, CREB-regulated transcription was in an ERK-dependent manner. Inhibition of ERK attenuated the DNA-binding efficiency of CREB to BDNF promoter. In addition, dendrite growth was improved after LLLT, characterized by upregulation of Rac1 activity and PSD-95 expression, and the increase in length, branching, and spine density of dendrites in hippocampal neurons. Together, these studies suggest that upregulation of BDNF with LLLT by activation of ERK/CREB pathway can ameliorate Aβ-induced neurons loss and dendritic atrophy, thus identifying a novel pathway by which LLLT protects against Aβ-induced neurotoxicity. Our research may provide a feasible therapeutic approach to control the progression of AD.
Edited by lostfalco, 11 February 2014 - 09:12 PM.
#43
Posted 30 March 2014 - 02:43 PM
J Neurosci. 1999 May 15;19(10):3681-90.
Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain.
Abstract
We have identified a mammalian opsin, encephalopsin, that shows strong and specific expression in the brain. Encephalopsin defines a new family of opsins and shows highest homology to vertebrate retinal and pineal opsins. Encephalopsin is highly expressed in the preoptic area and paraventricular nucleus of the hypothalamus, both regions implicated in encephalic photoreception in nonmammalian vertebrates. In addition,encephalopsin shows highly patterned expression in other regions of the brain, being enriched in selected regions of the cerebral cortex, cerebellar Purkinje cells, a subset of striatal neurons, selected thalamic nuclei, and a subset of interneurons in the ventral horn of the spinal cord. Rostrocaudal gradients of encephalopsin expression are present in the cortex, cerebellum, and striatum. Radial stripes of encephalopsin expression are seen in the cerebellum. In the cortex and cerebellum, encephalopsin expression is considerably higher and more highly patterned in the adult than in the neonate.Encephalopsin is the first putative extraocular opsin identified in mammals and may play a role in encephalic photoreception.
Edited by lostfalco, 30 March 2014 - 02:49 PM.
#44
Posted 30 March 2014 - 02:51 PM
http://www.ncbi.nlm....pubmed/21425483
J Integr Neurosci. 2011 Mar;10(1):65-88.
Emission of mitochondrial biophotons and their effect on electrical activity of membrane via microtubules.
Rahnama M1, Tuszynski JA, Bókkon I, Cifra M, Sardar P, Salari V.
Abstract
In this paper we argue that, in addition to electrical and chemical signals propagating in the neurons of the brain, signal propagation takes place in the form of biophoton production. This statement is supported by recent experimental confirmation of photon guiding properties of a single neuron. We have investigated the interaction of mitochondrial biophotons with microtubules from a quantum mechanical point of view. Our theoretical analysis indicates that the interaction of biophotons and microtubules causes transitions/fluctuations of microtubules between coherent and incoherent states. A significant relationship between the fluctuation function of microtubules and alpha-EEG diagrams is elaborated on in this paper. We argue that the role of biophotons in the brain merits special attention.
Edited by lostfalco, 30 March 2014 - 02:54 PM.
#45
Posted 30 March 2014 - 02:59 PM
PLoS One. 2014 Jan 15;9(1):e85643. doi: 10.1371/journal.pone.0085643. eCollection 2014.
Spatiotemporal imaging of glutamate-induced biophotonic activities and transmission in neural circuits.
Tang R1, Dai J2.
Author information
Abstract
The processing of neural information in neural circuits plays key roles in neural functions. Biophotons, also called ultra-weak photon emissions (UPE), may play potential roles in neural signal transmission, contributing to the understanding of the high functions of nervous system such as vision, learning and memory, cognition and consciousness. However, the experimental analysis of biophotonic activities (emissions) in neural circuits has been hampered due to technical limitations. Here by developing and optimizing an in vitro biophoton imaging method, we characterize the spatiotemporal biophotonic activities and transmission in mouse brain slices. We show that the long-lasting application of glutamate to coronal brain slices produces a gradual and significant increase of biophotonic activities and achieves the maximal effect within approximately 90 min, which then lasts for a relatively long time (>200 min). The initiation and/or maintenance of biophotonic activities by glutamate can be significantly blocked by oxygen and glucose deprivation, together with the application of a cytochrome c oxidase inhibitor (sodium azide), but only partly by an action potential inhibitor (TTX), an anesthetic (procaine), or the removal of intracellular and extracellular Ca(2+). We also show that the detected biophotonic activities in the corpus callosum and thalamus in sagittal brain slices mostly originate from axons or axonal terminals of cortical projection neurons, and that the hyperphosphorylation of microtubule-associated protein tau leads to a significant decrease of biophotonic activities in these two areas. Furthermore, the application of glutamate in the hippocampal dentate gyrus results in increased biophotonic activities in its intrahippocampal projection areas. These results suggest that the glutamate-induced biophotonic activities reflect biophotonic transmission along the axons and in neural circuits, which may be a new mechanism for the processing of neural information.
Edited by lostfalco, 30 March 2014 - 03:04 PM.
#46
Posted 18 September 2014 - 01:46 AM
Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis.
Transcranial low-level laser (light) therapy (LLLT) is a new non-invasive approach to treating a range of brain disorders including traumatic brain injury (TBI). We (and others) have shown that applying near-infrared light to the head of animals that have suffered TBI produces improvement in neurological functioning, lessens the size of the brain lesion, reduces neuroinflammation, and stimulates the formation of new neurons. In the present study we used a controlled cortical impact TBI in mice and treated the mice either once (4 h post-TBI, 1-laser), or three daily applications (3-laser) with 810 nm CW laser 36 J/cm2 at 50 mW/cm2 . Similar to previous studies, the neurological severity score improved in laser-treated mice compared to untreated TBI mice at day 14 and continued to further improve at days 21 and 28 with 3-laser being better than 1-laser. Mice were sacrificed at days 7 and 28 and brains removed for immunofluorescence analysis. Brain-derived neurotrophic factor (BDNF) was significantly upregulated by lasertreatment in the dentate gyrus of the hippocampus (DG) and the subventricular zone (SVZ) but not in the perilesional cortex (lesion) at day 7 but not at day 28. Synapsin-1 (a marker for synaptogenesis, the formation of new connections between existing neurons) was significantly upregulated in lesion and SVZ but not DG, at 28 days but not 7 days. The data suggest that the benefit of LLLT to the brain is partly mediated by stimulation of BDNFproduction, which may in turn encourage synaptogenesis. Moreover the pleiotropic benefits of BDNF in the brain suggest LLLT may have wider applications to neurodegenerative and psychiatric disorders. Neurological Severity Score (NSS) for TBI mice.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Edited by lostfalco, 18 September 2014 - 01:47 AM.
#47
Posted 22 September 2014 - 03:56 PM
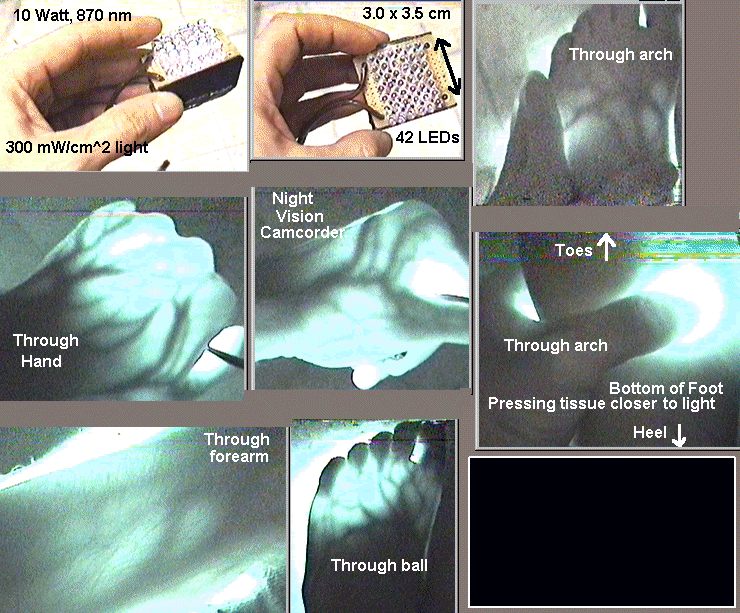
Right, heat in both the circuit and tissue is the limiting factor on applying light therapy. All manufacturers I've run into want to tell you 2 to 15 minutes is the treatment, but that's mostly out of hope and not knowing how much skin blocks. Few devices will do any good beneath 1 cm but definitely 1 inch is beyond all hope unless it is a 4 hour treatment. This is the ugly secret manufacturers will not tell you, and even lie (or more likely simply not know it's not true) that somehow infrared is magic and can go more than 1 inch. Shine the strongest infrared light through your palm and you'll have to be in a completely dark room for an infrared camera to barely see anything (like may attachment) On a bald person, the brain will be the easiest thing to treat, other than arthritic fingers. It's only 1 cm, mostly through skull bone, which according to the papers I'm seeing, is easier to penetrate than fat and much easier than skin, possibly because of too much melanin in skin and blood in fat. I'm going to test the numbers I have for fat because I find it hard to believe. Anyway, I don't expect pulsing to be better except for enabling stronger pulses with less heat which means deeper penetration and less treatment time but only if pulsing is timed to be utilized by the CCO more efficiently by having the off times than allow the CCO time to reset into modes that will be more receptive to the light. My son hurt his foot this morning and my pulsing device seemed to do a lot better than my more powerful (wattage) constant devices. I'm including a picture below, but it was in a dark room with a night vision camcorder, so do not thing the intensity that made it through was strong, even though it was with a device that is 10 times more powerful than the typical LED units.
this is way above my head but i will try to understand because i use infrared light for treating my skin.
you are saying that infrared light doesnt penetrate the skin more than 1cm? that so far the only good thing that infrared light penetrates deeply is the brain?
i bought like $20 lamp and also a seperate 250watt infrared light bulb! i bought 3 of them so I surround my body so all the infrared light hits my body in all directions.....i usually do this for 30 minutes a day....so this is worthless to do since infrared doesnt penetrate the skin more than 1cm?
#48
Posted 05 April 2015 - 12:04 AM
:O LLLT and DNA repair?
http://www.ncbi.nlm....pubmed/24930134
Although red laser lights lie in the region of non-ionizing radiations in the electromagnetic spectrum, there are doubts whether absorption of these radiations causes lesions in the DNA molecule. Our aim was to investigate the expression of the genes involved with base excision and nucleotide excision repair pathways in skin tissue submitted to burn injury and exposed to low-level red laser. Wistar rats were divided as follows: control group-rats burned and not irradiated, laser group-rats burned and irradiated 1 day after injury for five consecutive days, and later laser group-rats injured and treated 4 days after injury for five consecutive days. Irradiation was performed according to a clinical protocol (20 J/cm(2), 100 mW, continuous wave emission mode). The animals were sacrificed on day 10, and scarred tissue samples were withdrawn for total RNA extraction, complementary DNA(cDNA) synthesis, and evaluation of gene expression by quantitative polymerase chain reaction. Low-level red laser exposure (1) reduces the expression of APE1 messenger (mRNA), (2) increases the expression of OGG1 mRNA, (3) reduces the expression of XPC mRNA, and (4) increases the expression of XPA mRNA both in laser and later laser groups. Red laser exposure at therapeutic fluences alters the expression of genes related to base excision and nucleotide excision pathways of DNA repair during wound healing of burned skin.
http://www.ncbi.nlm....pubmed/22941447
Special properties of laser light have led to its usefulness in many applications in therapy. Excitation of endogenous chromophores in biotissues and generation of free radicals could be involved in its biological effects. DNA lesions induced by free radicals are repaired by base excision repairpathway. In this work, we evaluated the expression of APE1 and OGG1 genes related to repair of DNA lesions induced by free radicals. Skin and muscle tissues of Wistar rats were exposed to low-intensity infrared laser at different fluences and frequencies. After laser exposition of 1 and 24 h, tissue samples were withdrawn for total RNA extraction, cDNA synthesis, and evaluation of APE1 and OGG1 gene expression by quantitative polymerase chain reaction. Data obtained show that laser radiation alters the expression of APE1 and OGG1 mRNA differently in skin and muscle tissues of Wistar rats depending of the fluence, frequency, and time after exposure. Our study suggests that low-intensity infrared laser affects expression of genes involved in repair of DNA lesions by base excision repair pathway.
http://www.ncbi.nlm....pubmed/25207838
Superpulsed (Ga-As, 904 nm) low-level laser therapy (LLLT) attenuates inflammatory response and enhances healing of burn wounds.Low-level laser therapy (LLLT) using superpulsed near-infrared light can penetrate deeper in the injured tissue and could allow non-pharmacological treatment for chronic wound healing. This study investigated the effects of superpulsed laser (Ga-As 904 nm, 200 ns pulse width; 100 Hz; 0.7 mW mean output power; 0.4 mW/cm2 average irradiance; 0.2 J/cm2 total fluence) on the healing of burn wounds in rats, and further explored the probable associated mechanisms of action. Irradiated group exhibited enhanced DNA, total protein, hydroxyproline and hexosamine contents compared to the control and silver sulfadiazine (reference care) treated groups. LLLT exhibited decreased TNF-α level and NF-kB, and up-regulated protein levels of VEGF, FGFR-1, HSP-60, HSP-90, HIF-1α and matrix metalloproteinases-2 and 9 compared to the controls. In conclusion, LLLT using superpulsed 904 nm laser reduced the inflammatory response and was able to enhance cellular proliferation, collagen deposition and wound contraction in therepair process of burn wounds. Photomicrographs showing no, absence inflammation and faster wound contraction in LLLT superpulsed (904 nm)laser treated burn wounds as compared to the non-irradiated control and silver sulfadiazine (SSD) ointment (reference care) treated wounds.
Edited by BieraK, 05 April 2015 - 12:08 AM.
#49
Posted 09 May 2015 - 03:12 AM
http://www.ncbi.nlm....pubmed/15302557
J Theor Biol. 2004 Sep 21;230(2):261-70.
Propagation of electromagnetic radiation in mitochondria?Mitochondria are the main source of ultra-weak chemiluminescence generated by reactive oxygen species, which are continuously formed during the mitochondrial oxidative metabolism. Vertebrate cells show typically filamentous mitochondria associated with the microtubules of the cytoskeleton, forming together a continuous network (mitochondrial reticulum). The refractive index of both mitochondria and microtubules is higher than the surrounding cytoplasm, which results that the mitochondrial reticulum can act as an optical waveguide, i.e. electromagnetic radiation can propagate within the network. A detailed analysis of the inner structure of mitochondria shows, that they can be optically modelled as a multi-layer system with alternating indices of refraction. The parameters of this multi-layer system are dependent on the physiologic state of the mitochondria. The effect of the multi-layer system on electromagnetic radiation propagating along the mitochondrial reticulum is analysed by the transfer-matrix method. If induced light emission could take place in mitochondria, the multi-layer system could lead to lasing action like it has been realized in technical distributed feedback laser. Based on former reports about the influence of external illumination on the physiology of mitochondria it is speculated whether there exists some kind of long-range interaction between individual mitochondria mediated by electromagnetic radiation.
#50
Posted 27 May 2015 - 02:56 AM
http://www.ncbi.nlm....pubmed/26005853
Med Sci Monit. 2015 May 25;21:1507-11. doi: 10.12659/MSM.894481.
Mitochondria and chloroplasts shared in animal and plant tissues: significance of communication.
Mitochondria have long been recognized as the main source of energy production for the eukaryotic cell. Recent studies have found that the mitochondria have a variety of dynamic functions aside from the production of energy. It communicates bidirectionally with other organelles in order to modulate its energy balance efficiently, as well as maintain homeostasis, ultimately prolonging its own and the cell's longevity. The mitochondriaachieves this level of regulation via specific and common bidirectional chemical messengers, especially involving the endoplasmic/sarcoplasmic reticulum (ER/SR), deoxyribonucleoside triphosphates (dNTP's), ATP and the generation of reactive oxygen species (ROS). Its communication network is also involved in stress associated events. In this regard, the activation of the Bax family proteins and the release of cytochrome c occurs during cellular stress. The communication can also promote apoptosis of the cell. When mitochondrial abnormalities cannot be dealt with, there is an increased chance that major illnesses like type 2 diabetes, Alzheimer's disease, and cancer may occur. Importantly, functioning chloroplasts can be found in animals, suggesting conserved chemical messengers during its evolutionary path. The dynamic capacity of mitochondria is also noted by their ability to function anaerobically. Indeed, this latter phenomenon may represent a return to an earlier developmental stage of mitochondria, suggesting certain disorders result from its untimely appearance.
http://www.ncbi.nlm....pubmed/24606795
Curr Pharm Des. 2014;20(35):5507-9.
Mitochondrial biogenesis: pharmacological approaches.
Organelle biogenesis is concomitant to organelle inheritance during cell division. It is necessary that organelles double their size and divide to give rise to two identical daughter cells. Mitochondrial biogenesis occurs by growth and division of pre-existing organelles and is temporally coordinated with cell cycle events [1]. However, mitochondrial biogenesis is not only produced in association with cell division. It can be produced in response to an oxidative stimulus, to an increase in the energy requirements of the cells, to exercise training, to electrical stimulation, to hormones, during development, in certain mitochondrial diseases, etc. [2]. Mitochondrial biogenesis is therefore defined as the process via which cells increase their individual mitochondrial mass [3]. Recent discoveries have raised attention to mitochondrial biogenesis as a potential target to treat diseases which up to date do not have an efficient cure. Mitochondria, as the major ROS producer and the major antioxidant producer exert a crucial role within the cell mediating processes such as apoptosis, detoxification, Ca2+ buffering, etc. This pivotal role makes mitochondria a potential target to treat a great variety of diseases. Mitochondrial biogenesis can be pharmacologically manipulated. This issue tries to cover a number of approaches to treat several diseases through triggering mitochondrial biogenesis. It contains recent discoveries in this novel field, focusing on advanced mitochondrial therapies to chronic and degenerative diseases, mitochondrial diseases, lifespan extension, mitohormesis, intracellular signaling, new pharmacological targets and natural therapies. It contributes to the field by covering and gathering the scarcely reported pharmacological approaches in the novel and promising field of mitochondrial biogenesis. There are several diseases that have a mitochondrial origin such as chronic progressive external ophthalmoplegia (CPEO) and the Kearns- Sayre syndrome (KSS), myoclonic epilepsy with ragged-red fibers (MERRF), mitochondrial encephalomyopathy, lactic acidosis and strokelike episodes (MELAS), Leber's hereditary optic neuropathy (LHON), the syndrome of neurogenic muscle weakness, ataxia and retinitis pigmentosa (NARP), and Leigh's syndrome. Likewise, other diseases in which mitochondrial dysfunction plays a very important role include neurodegenerative diseases, diabetes or cancer. Generally, in mitochondrial diseases a mutation in the mitochondrial DNA leads to a loss of functionality of the OXPHOS system and thus to a depletion of ATP and overproduction of ROS, which can, in turn, induce further mtDNA mutations. The work by Yu-Ting Wu, Shi-Bei Wu, and Yau-Huei Wei (Department of Biochemistry and Molecular Biology, National Yang-Ming University, Taiwan) [4] focuses on the aforementioned mitochondrial diseases with special attention to the compensatory mechanisms that prompt mitochondria to produce more energy even under mitochondrial defect-conditions. These compensatory mechanisms include the overexpression of antioxidant enzymes, mitochondrial biogenesis and overexpression of respiratory complex subunits, as well as metabolic shift to glycolysis. The pathways observed to be related to mitochondrial biogenesis as a compensatory adaptation to the energetic deficits in mitochondrial diseases are described (PGC- 1, Sirtuins, AMPK). Several pharmacological strategies to trigger these signaling cascades, according to these authors, are the use of bezafibrate to activate the PPAR-PGC-1α axis, the activation of AMPK by resveratrol and the use of Sirt1 agonists such as quercetin or resveratrol. Other strategies currently used include the addition of antioxidant supplements to the diet (dietary supplementation with antioxidants) such as L-carnitine, coenzyme Q10,MitoQ10 and other mitochondria-targeted antioxidants,N-acetylcysteine (NAC), vitamin C, vitamin E vitamin K1, vitamin B, sodium pyruvate or -lipoic acid. As aforementioned, other diseases do not have exclusively a mitochondrial origin but they might have an important mitochondrial component both on their onset and on their development. This is the case of type 2 diabetes or neurodegenerative diseases. Type 2 diabetes is characterized by a peripheral insulin resistance accompanied by an increased secretion of insulin as a compensatory system. Among the explanations about the origin of insulin resistance Mónica Zamora and Josep A. Villena (Department of Experimental and Health Sciences, Universitat Pompeu Fabra / Laboratory of Metabolism and Obesity, Universitat Autònoma de Barcelona, Spain) [5] consider the hypothesis that mitochondrial dysfunction, e.g. impaired (mitochondrial) oxidative capacity of the cell or tissue, is one of the main underlying causes of insulin resistance and type 2 diabetes. Although this hypothesis is not free of controversy due to the uncertainty on the sequence of events during type 2 diabetes onset, e.g. whether mitochondrial dysfunction is the cause or the consequence of insulin resistance, it has been widely observed that improving mitochondrial function also improves insulin sensitivity and prevents type 2 diabetes. Thus restoring oxidative capacity by increasing mitochondrial mass appears as a suitable strategy to treat insulin resistance. The effort made by researchers trying to understand the signaling pathways mediating mitochondrial biogenesis has uncovered new potential pharmacological targets and opens the perspectives for the design of suitable treatments for insulin resistance. In addition some of the current used strategies could be used to treat insulin resistance such as lifestyle interventions (caloric restriction and endurance exercise) and pharmacological interventions (thiazolidinediones and other PPAR agonists, resveratrol and other calorie restriction mimetics, AMPK activators, ERR activators). Mitochondrial biogenesis is of special importance in modern neurochemistry because of the broad spectrum of human diseases arising from defects in mitochondrial ion and ROS homeostasis, energy production and morphology [1]. Parkinson´s Disease (PD) is a very good example of this important mitochondrial component on neurodegenerative diseases. Anuradha Yadav, Swati Agrawal, Shashi Kant Tiwari, and Rajnish K. Chaturvedi (CSIR-Indian Institute of Toxicology Research / Academy of Scientific and Innovative Research, India) [6] remark in their review the role of mitochondrial dysfunction in PD with special focus on the role of oxidative stress and bioenergetic deficits. These alterations may have their origin on pathogenic gene mutations in important genes such as DJ-1, -syn, parkin, PINK1 or LRRK2. These mutations, in turn, may cause defects in mitochondrial dynamics (key events like fission/fusion, biogenesis, trafficking in retrograde and anterograde directions, and mitophagy). This work reviews different strategies to enhance mitochondrial bioenergetics in order to ameliorate the neurodegenerative process, with an emphasis on clinical trials reports that indicate their potential. Among them creatine, Coenzyme Q10 and mitochondrial targeted antioxidants/peptides are reported to have the most remarkable effects in clinical trials. They highlight a dual effect of PGC-1α expression on PD prognosis. Whereas a modest expression of this transcriptional co-activator results in positive effects, a moderate to substantial overexpession may have deleterious consequences. As strategies to induce PGC-1α activation, these authors remark the possibility to activate Sirt1 with resveratrol, to use PPAR agonists such as pioglitazone, rosiglitazone, fenofibrate and bezafibrate. Other strategies include the triggering of Nrf2/antioxidant response element (ARE) pathway by triterpenoids (derivatives of oleanolic acid) or by Bacopa monniera, the enhancement of ATP production by carnitine and -lipoic acid. Mitochondrial dysfunctions are the prime source of neurodegenerative diseases and neurodevelopmental disorders. In the context of neural differentiation, Martine Uittenbogaard and Anne Chiaramello (Department of Anatomy and Regenerative Biology, George Washington University School of Medicine and Health Sciences, USA) [7] thoroughly describe the implication of mitochondrial biogenesis on neuronal differentiation, its timing, its regulation by specific signaling pathways and new potential therapeutic strategies. The maintenance of mitochondrial homeostasis is crucial for neuronal development. A mitochondrial dynamic balance is necessary between mitochondrial fusion, fission and quality control systems and mitochondrial biogenesis. Concerning the signaling pathways leading to mitochondrial biogenesis this review highlights the implication of different regulators such as AMPK, SIRT1, PGC-1α, NRF1, NRF2, Tfam, etc. on the specific case of neuronal development, providing examples of diseases in which these pathways are altered and transgenic mouse models lacking these regulators. A common hallmark of several neurodegenerative diseases (Huntington´s Disease, Alzheimer´s Disease and Parkinson´s Disease) is the impaired function or expression of PGC-1α, the master regulator of mitochondrial biogenesis. Among the promising strategies to ameliorate mitochondrial-based diseases these authors highlight the induction of PGC-1α via activation of PPAR receptors (rosiglitazone, bezafibrate) or modulating its activity by AMPK (AICAR, metformin, resveratrol) or SIRT1 (SRT1720 and several isoflavone-derived compounds). This article also presents a review of the current animal and cellular models useful to study mitochondriogenesis. Although it is known that many neurodegenerative and neurodevelopmental diseases are originated in mitochondria, the regulation of mitochondrial biogenesis has never been extensively studied. (ABSTRACT TRUNCATED)
Edited by lostfalco, 27 May 2015 - 02:59 AM.
#51
Posted 27 May 2015 - 04:32 AM
Did you see this item Falco?
Scientists have a crystal ball on their hands: bursts of activity in the energy-producing mitochondria in a worm’s cells accurately predict how long it will live.
The findings, published today in Nature1, suggest that an organism’s lifespan is, for the most part, predictable in early adulthood. Unlike other biomarkers for ageing, which work under limited conditions, these mitochondrial bursts are a stable predictor for a variety of genetic, environmental and developmental histories. “Mitochondrial flashes have an amazing power to predict the remaining lifespan in animals,” says study lead Meng-Qiu Dong, a geneticist who studies ageing in the Caenorhabditis elegans worm at the National Institute of Biological Sciences in Beijing. “There is truth in the mitochondrial theory of ageing.”
The mitochondria are organelles that power the cells of plants, animals and other eukaryotic organisms. During energy production, they produce reactive oxygen molecules, such as free radicals, that can cause stress and damage the mitochondria. Although mitochondria break down over time, the mitochondrial theory of ageing, first proposed2 in 1972, remains controversial and unproven. For instance, some long-lived organisms, such as naked mole rats, endure with high levels of oxidative damage. Nevertheless, many scientists think that mitochondria remain the primary drivers of ageing.
Swan song
Related storiesDong became interested in the 2008 discovery3 that mitochondria produce reactive oxygen molecules in 10-second pulses — ‘mitoflashes’ — every couple minutes. For the first time, scientists could observe individual mitochondria and their rates of activty through the course of an animal’s life. In this study, Dong initially compared mitoflash rates in short-lived C. elegans worms, which live an average of 21 days, to long-lived worms that live an average of 30 days or more. She found that, in all of the animals, there were two moments in life when mitoflashes bunched closely together: one burst during early adulthood and another during senescence.
At first, she expected that the burst later in life would be the important one. “It was a total failure,” she says. Instead, it was the early burst that revealed a correlation between flash frequency and lifespan: worms with an average lifespan of 21 days had more frequent flashes during this burst than their longer-lived brethren. The correlation held across 29 genetic mutants with various lifespans.
Mitoflashes also proved to be a powerful record of a worm’s early life experiences. For instance, worms exposed to heat shock or starvation tend to have longer lives, and predictably, their mitoflashes occurred at longer intervals. Even genetically identical worms that had different lifespans due to chance events alone showed the same correlation between mitoflash frequency and longevity. The most striking finding came when Dong treated a long-lived worm to increase its production of reactive oxygen molecules. This shortened the worm’s life and increased the rate of mitoflashes.
“It’s a great paper,” says Jan Gruber, a biogerontologist at the Yale-National University of Singapore College. He had expected to see a gradual decline in mitoflashes over time, and was particularly surprised by the burst that comes towards the end of life, a kind of swan song. “We know that the mitochondria shut down with ageing,” he says. “Why do they shut down in a dramatic fashion?”
#52
Posted 27 May 2015 - 12:39 PM
Did you see this item Falco?
No, I haven't seen that before. Thanks, Cosmic.
#53
Posted 28 May 2015 - 04:19 AM
This sounds like an interesting article. I can't get it via my database subscription, can anyone access it?
Curr Aging Sci. 2015 May 19. [Epub ahead of print]Mitochondrial pharmaceutics: A new therapeutic strategy to ameliorate oxidative stress in Alzheimer's disease.Author information
- 1Department of Biochemistry Amala Institute of Medical Sciences Amala Nagar, Thrissur-680 555, Kerala, India. taajith@rediffmail.com.
AbstractAssociation between amyloid-β (Aβ) toxicity, mitochondrial dysfunction, oxidative stress and neuronal damage has been demonstrated in the pathophysiology of Alzheimer's disease (AD). In the early stages of the disease, the defect in energy metabolism was found to be severe. This may probably due to the Aβ and ROS-induced declined activity of complexes in electron transport chain (ETC) as well as damages to mitochondrial DNA. Though clinically inconclusive, supplementation with antioxidants are reported to be beneficial especially in the early stages of the disease. A mild to moderate improvement in dementia is possible with therapy using antioxidants viz coenzyme Q10 (ubiquinone), α-lipoic acid, selenium, omega-3 fatty acids and vitamin E, emphasize their possible role as adjuvant with the existing conventional treatment. Since mitochondrial dysfunction has been observed, a new therapeutic strategy called 'Mitochondrial Medicine' which is aimed to maintain the energy production as well as to ameliorate the enhanced apoptosis of nerve cells has been developed. Mitochondrial CoQ10, Szeto-Schiller peptide-31 and superoxide dismutase/catalase mimetic, EUK-207 were the mitochondrial targeted agents demonstrated in experimental studies. This article discusses the mitochondrial impairment and the possible mitochondria targeted therapeutic intervention in AD.
http://www.ncbi.nlm....pubmed/25986626
#54
Posted 30 September 2015 - 05:14 PM
I posted the free full text to this amazing study here: http://www.longecity...-84#entry742915
http://www.ncbi.nlm....pubmed/26187720
Trends Neurosci. 2015 Aug;38(8):468-74. doi: 10.1016/j.tins.2015.06.001. Epub 2015 Jul 15.
Mitochondrial synapses: intracellular communication and signal integration.
Communication is a central theme in biology. Consequently, specialized structures have evolved to permit rapid communication among cells, tissues, organs, and physiological systems, thus enhancing the overall function and adaptation of the organism. A prime example is the neuronal synapse. In the brain, synaptic communication establishes neuronal networks with the capacity to integrate, process, and store information, giving rise to complex output signals capable of orchestrating functions across the organism. At the intracellular level, discoveries now reveal the existence of 'mitochondrial synapses' establishing mitochondrial networks, with defined chromatin-modifying mitochondrial output signals capable of orchestrating gene expression across the genome. These discoveries raise the possibility that in addition to their role as powerhouses and neuromodulators, mitochondria behave as intracellular signal-processing networks.
Highlights
•Signal processing by brain neurons arises from synaptic connections.
•Mitochondrial networks transmit information analogous to brain neuronal networks.
•Information is exchanged between mitochondria via ‘mitochondrial synapses’.
•Chromatin-remodeling mitochondrial signals impact nuclear gene expression.
•Mitochondria are neuromodulators, impacting presynaptic neurotransmitter release.
Edited by lostfalco, 30 September 2015 - 05:25 PM.
#55
Posted 13 November 2015 - 06:06 AM
http://www.ncbi.nlm....pubmed/26336891
J Integr Neurosci. 2015 Sep;14(3):419-29. doi: 10.1142/S0219635215300012. Epub 2015 Sep 4.
Ultraweak photon emission in the brain.Besides the low-frequency electromagnetic body-processes measurable through the electroencephalography (EEG), electrocardiography (ECG), etc. there are processes that do not need external excitation, emitting light within or close to the visible spectra. Such ultraweak photon emission (UPE), also named biophoton emission, reflects the cellular (and body) oxidative status. Recently, a growing body of evidence shows that UPE may play an important role in the basic functioning of living cells. Moreover, interesting evidences are beginning to emerge that UPE may well play an important role in neuronal functions. In fact, biophotons are byproducts in cellular metabolism and produce false signals (e.g., retinal discrete dark noise) but on the other side neurons contain many light sensitive molecules that makes it hard to imagine how they might not be influenced by UPE, and thus UPE may carry informational contents. Here, we investigate UPE in the brain from different points of view such as experimental evidences, theoretical modeling, and physiological significance.
Also tagged with one or more of these keywords: tulip
 |
Community →
LongeCity →
Forum Issues →
FAQ- Please Read! →
₮ulips?Started by caliban , 18 Aug 2016 |
|

|
|
Science & Health →
Brain Health →
Getting Back ON TrackStarted by Q did it! , 16 Aug 2015 |
|

|
||
Science & Health →
Brain Health →
Mind helping with my stack?Started by Yume , 06 Mar 2015 |
|

|
||
Science & Health →
Supplements →
Critique my LifeStarted by Awareness , 02 Oct 2014 |
|

|
||
Science & Health →
Brain Health →
Nootropic Stacks →
TULIP. Are people still doing it?Started by LongecityM20 , 26 May 2014 |
|

|
2 user(s) are reading this topic
0 members, 2 guests, 0 anonymous users














































