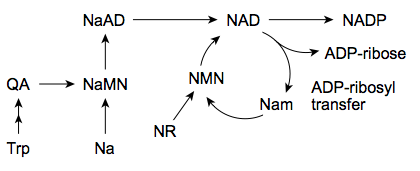
Main questions to me are
1) how much oral NR needs to be taken in one sitting to saturate the hydrolysis/phosphorylysis of NR in the intestine
2) what happens to additional oral NR, is it broken down to something other than niacinamide, or does it passively diffuse through the intestine, or whatnot.
3) If it diffuse through the intestine as is, does it survive the liver first past metabolism?
4) If it does, does the high level of NR in blood translate into levels of NAD in cells higher that can reasonably be obtained from niacin. For example this study doesnt look too good for NR (against niacin) - eg looking at graph D in figure 1
5) If significant NR reach the blood, does its metabolism require methyl groups (like that of niacin and niacinamide)
6) If oral NR does not beat niacin, is there other delivery methods that works better. It looks like many people here use sublingual niagen.
Depending on the answers to those questions, an efficient strategy to take NR might be to take a very high dose once a week or once a month, instead of taking it daily. Or daily sublingual. Or infusions. And similar to niacin and niacinamide, making sure one's methylation is working well.
Btw what do we know about the experiment in which Sinclair used gavage? Did it find that "oral dosing clearly works"?































 This topic is locked
This topic is locked