Immuno-oncology represents an innovative approach to cancer research that seeks to harness the body's own immune system to fight tumor cells. I though I would add info on the drugs of new and in my first post, drugs of old
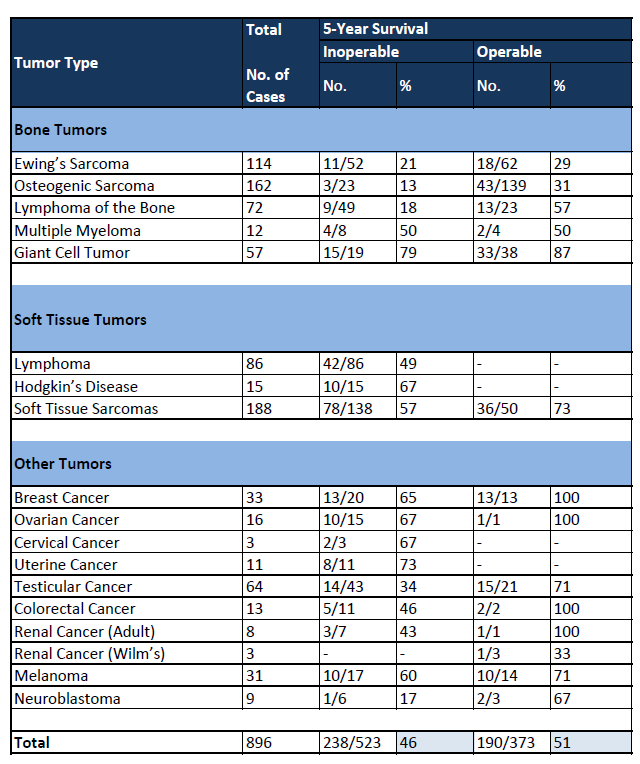
Dr William Coley MD was an American orthopedic (bone) surgeon, surgical oncologist and cancer researcher, who was a pioneer of cancer immunotherapy. He began his career as a surgeon at New York Cancer Hospital, which later became part of the Memorial Sloan-Kettering Cancer Center. He became more interested in cancer treatment when one of his early patients, Elizabeth Dashiell, died from her sarcoma. While going through hospital records, he found a sarcoma case study of one patient, Fred Stein whose tumor disappeared following a high fever from a bacterial erysipelas infection, which is now known as Streptococcus pyogenes. Mr. Stein's seemingly miraculous cure contrasted with Bessie Dashiell's rapid death and inspired Coley to scour the literature looking for other patients who had cancer remission due to a concurrent bacterial infection. He was aware of anecdotal theories of the beneficial effect of fever on malignant tumors. In addition to these anecdotes, he was able to find specific examples in the literature. Dr Coley was able to find approximately 47 cases in the literature documenting the beneficial effect of infections on tumors. He was convinced that having a severe infection could cause cancer to regress. It took a great deal of courage, but in 1891 he injected his first patient with streptococcal organisms and noticed the shrinkage of a malignant tumor. This encouraged him to treat two other patients with long-bone sarcomas. The injections appeared to be quite dangerous, and two of his patients died of infection. However, there was some observable shrinkage of their malignant tumors. He published his first work describing these three patients in 1891. Because of the danger of live streptococcal organisms, he continued his treatments using a heat-killed streptococcal organism combined with a second organism that is known as Serratia marcescens. This concoction became known as Coley's Toxin. By 1893, he had tried his toxin on ten patients, most of whom did well. By 1916, he had documented 80 more cases in a monograph. By the end of his career, he had written over 150 papers on this subject and treated almost 1,000 cases. Dr William Coley's intuitions were correct, stimulating the immune system may be effective in treating cancer. He was a model of the clinician-scientist, treating patients and using his practice to initiate research and build theories. But he was a man before his time, and he met with severe criticism. Despite this criticism, however, Coley stuck with his ideas, and today we are recognizing their potential value. A scientific review of 897 cancer patients treated with various formulations of Coley Fluid up to 110 years ago found that complete regression and 5-year survival occurred in 46% of the 523 inoperable cases and 51% of the 374 operable cases. These results are comparable to modern 5-year survival rates. The National Cancer Institute estimates overall 5-year cancer survival at 35% in 1950-54 and 63.8% in 1992-98. To determine comparable rates of 10-year survival, in 1999 researchers compared historical Coley Fluid patients with matched controls from the National Cancer Institute Surveillance Epidemiology End Result database. The study found higher rates of 10-year survival for Coley Fluid patients compared to modern patients in kidney cancer (33% to 23%), ovarian cancer (55% to 29%) and sarcoma (50% to 38%). ''For more than six years (2005-2012), MBVax Bioscience produced Coley Fluid and distributed it for compassionate use to countries where government regulators allowed its importation and use. The documented clinical responses of end-stage cancer patients treated according to the administration protocol show that Coley Fluid is a very effective cancer treatment with confirmed tumor regression in about 70% of cases and complete remission in about 20%. These results must be confirmed in clinical trials, but regulators in Canada, Europe and the United States have denied permission because Coley Fluid production does not meet EU or FDA manufacturing standard for pharmaceuticals. Meeting these standards will require a new production facility. When financing is available, MBVax Bioscience plans to build a new production facility, obtain regulatory certification and commence clinical trials.'' Sadly they haven't been able to get funding because investors don't believe them, nor the case reports.
More info here http://www.pvanuden....perhaps_21.html
A Phase I did take place, but cost over $1 million http://clincancerres...2-1116.full.pdfand Pubmed 22847809






























 This topic is locked
This topic is locked













