Source [1]
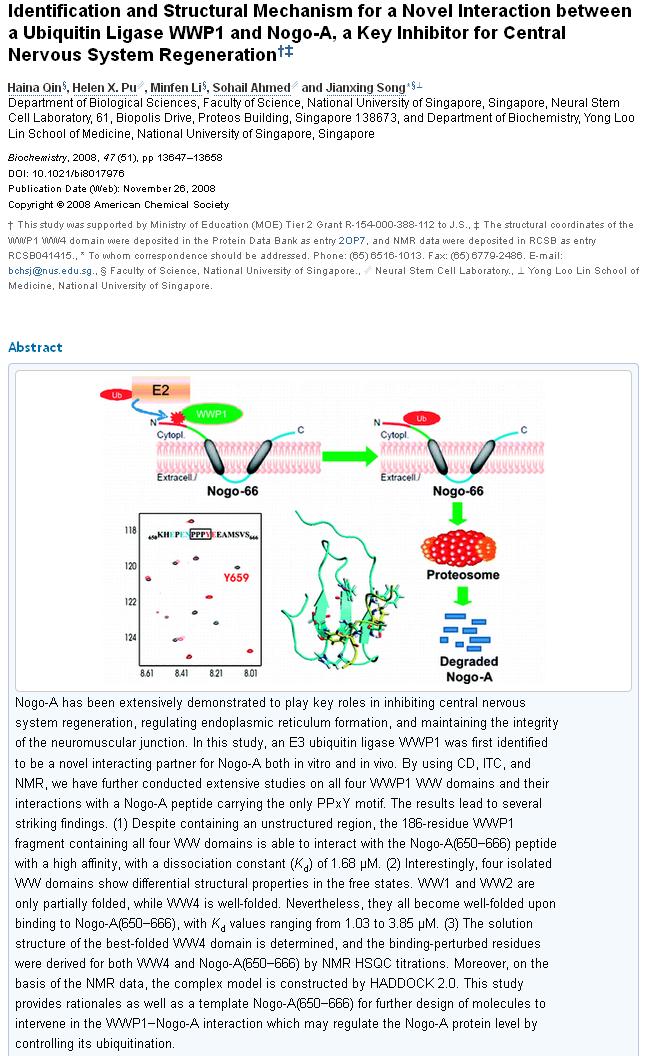
Each step in the ladder or pathway has potential to be exploited. The confines of this post are too brief to expound on every step. The Nogo-A is one example, which normally functions to inhibit axon growth:
Polyphenols from green tea prevent antineuritogenic action of Nogo-A via 67-kDa laminin receptor and hydrogen peroxide.
Gundimeda U1, McNeill TH, Barseghian BA, Tzeng WS, Rayudu DV, Cadenas E, Gopalakrishna R. (2015)
Axonal regeneration after injury to the CNS is hampered by myelin-derived inhibitors, such as Nogo-A. Natural products, such as green tea, which are neuroprotective and safe for long-term therapy, would complement ongoing various pharmacological approaches. In this study, using nerve growth factor-differentiated neuronal-like Neuroscreen-1 cells, we show that extremely low concentrations of unfractionated green tea polyphenol mixture (GTPP) and its active ingredient, epigallocatechin-3-gallate (EGCG), prevent both the neurite outgrowth-inhibiting activity and growth cone-collapsing activity of Nogo-66 (C-terminal domain of Nogo-A). Furthermore, a synergistic interaction was observed among GTPP constituents. This preventive effect was dependent on 67-kDa laminin receptor (67LR) to which EGCG binds with high affinity. The antioxidants N-acetylcysteine and cell-permeable catalase abolished this preventive effect of GTPP and EGCG, suggesting the involvement of sublethal levels of H2 O2 in this process. Accordingly, exogenous sublethal concentrations of H2 O2 , added as a bolus dose (5 μM) or more effectively through a steady-state generation (1-2 μM), mimicked GTPP in counteracting the action of Nogo-66. Exogenous H2 O2 mediated this action by bypassing the requirement of 67LR. Taken together, these results show for the first time that GTPP and EGCG, acting through 67LR and elevating intracellular sublethal levels of H2 O2 , inhibit the antineuritogenic action of Nogo-A. Currently, several agents are being evaluated for overcoming axonal growth inhibitors to promote functional recovery after stroke and spinal cord injury. Epigallocatechin-3-gallate (EGCG), present in green tea polyphenol mixture (GTPP), prevents antineuritogenic activity of Nogo-A, a myelin-derived axonal growth inhibitor. The preventive action of EGCG involves the cell-surface-associated 67-kDa laminin receptor and H2 O2 . GTPP may complement ongoing efforts to treat neuronal injuries.
Panax notoginseng saponins promotes stroke recovery by influencing expression of Nogo-A, NgR and p75NGF, in vitro and in vivo.
Liu L, Zhu L, Zou Y, Liu W, Zhang X, Wei X, Hu B, Chen J. (2014)
The spontaneous recovery of function after injury in the adult central nervous system is limited due to the several proteins, such as Nogo-A that have repulsive or inhibitory effects on growing neuritis. The Chinese herbal medicine extraction Panax notoginseng saponins (PNS) injection has been widely used and effective in repairing the function of impaired nerves, but the mechanism of this herbal medicine is still poorly understood. This project evaluated the effect of Panax notoginseng saponins on neurological functional recovery and on the expression of Nogo-A, NgR and p75 at 7, 14 and 28 d after middle cerebral artery occlusion (MCAO) in rats and also oxygen-glucose deprivation/reperfusion (OGD/R) model on SH-SY5Y cells. We found that the expression of Nogo-A, NgR and p75 of rats receiving MCAO surgery increased on the 7th day, reached a peak on the 14th or 28th day and maintained high levels and Panax notoginseng saponins significantly decreased these expressions. This may be the mechanism of Panax notoginseng saponins that contributes to the recovery of nerve function, which plays an important role in brain protection after cerebral infarction.
Effects of ephedrine on expression of Nogo-A and synaptophysin in neonatal rats following hypoxic-ischemic brain damage
Siyuan Chen, Nong Xiao, Xiaoping Zhang (2010)
BACKGROUND: Central nervous system axons regenerate poorly following neonatal hypoxic-ischemic brain damage (HIBD), partly due to inhibitors, such as Nogo-A. Very few studies have addressed the regulation of Nogo-A in neonatal rats following HIBD. However, numerous studies have shown that ephedrine accelerates neuronal remodeling and promotes recovery of neural function in neonatal rats following HIBD.OBJECTIVE: To investigate the effects of ephedrine on expression of Nogo-A and synaptophysin in brain tissues of neonatal rats following HIBD.
DESIGN, TIME AND SETTING: A completely randomized, controlled study was performed at the Immunohistochemistry Laboratory of the Research Institute of Pediatrics, Children's Hospital of Chongqing Medical University from August 2008 to March 2009.
MATERIALS: Ephedrine hydrochloride (Chifeng Pharmaceutical Group, China), rabbit anti-Nogo-A polyclonal antibody (Abcam, UK), and rabbit anti-synaptophysin polyclonal antibody (Lab Vision, USA) were used in this study.
METHODS: A total of 96 healthy, neonatal, Sprague Dawley rats were randomly assigned to three groups (n = 32): sham operation, HIBD, and ephedrine. The HIBD model was established by permanent occlusion of the left common carotid artery, followed by 2 hours of hypoxia (8% oxygen and 92% nitrogen). In the sham operation group, the left common carotid artery was exposed, but was not ligated or subjected to hypoxia. Rats in the ephedrine group were intraperitoneally injected with ephedrine immediately following HIBD, with 1.5 mg/kg each time. Rats in the sham operation and HIBD groups were injected with an equal volume of saline. All neonatal rats were treated once daily for 7 days.
MAIN OUTCOME MEASURES: Histopathological damage to the cortex and hippocampus was determined by hematoxylin-eosin staining. Expression of Nogo-A and synaptophysin was detected using immunohistochemical staining.
RESULTS: Neuronal degeneration and edema were observed in the hypoxic-ischemic cortex and hippocampus by hematoxylin-eosin staining. Compared with the sham operation group, the levels of Nogo-A significantly increased in the HIBD group at various time points (P < 0.01). Nogo-A expression was significantly reduced in the ephedrine group compared with the HIBD group (P < 0.01). Synaptophysin expression was significantly decreased in the hypoxic-ischemic cortex, compared with the sham operation group (P < 0.01). Synaptophysin levels were significantly increased in the ephedrine group, compared with the HIBD group (P < 0.01).
CONCLUSION: Altered Nogo-A expression was associated with inversely altered synaptophysin expression. The use of ephedrine normalized expression levels of Nogo-A and synaptophysin following HIBD.
Effects of curcumin on hippocampal expression of NgR and axonal regeneration in Aβ-induced cognitive disorder rats.
Yin HL1, Wang YL2, Li JF1, Han B1, Zhang XX1, Wang YT1, Geng S1. (2014)
Curcumin has been widely used for the prevention and treatment of Alzheimer's disease (AD), but its mechanism is still not clear. Inhibitory factors of axonal regeneration have been shown to cause a series of pathophysiological changes in the early period of AD. In this study, the co-receptor (Nogo receptor; NgR) of three axonal growth-inhibitory proteins was examined, and effects of curcumin on spatial learning and memory abilities and hippocampal axonal growth were investigated in amyloid β-protein (Aβ)1-40-induced AD rats. Results showed that the expression of NgR in the AD group significantly increased and the number of axonal protein-positive fibers significantly reduced. The spatial learning and memory abilities of AD rats were significantly improved in the curcumin group. Furthermore, hippocampal expressions of NgR mRNA and protein decreased, and the expression of axonal protein significantly increased. There was a negative correlation between the expression of NgR and axonal growth. Together, these results suggested that curcumin could improve the spatial learning and memory abilities of AD rats. The mechanism might be related with its lowering of hippocampal NgR expression and promoting axonal regeneration.
Ginseng gets brownie points for having more supporting evidence:
The Study on Regenerative Effects of Ginseng on Injured Axonal and Non-Neuronal cell
Lim, Chang-Bum (2014)
Objective: This study was carried out to understand effects of ginseng(hearinafter ; GS, Panax Ginseng) extract on regeneration responses on injured sciatic nerves in rats.
Methods: Using white mouse, we damaged sciatic nerve & central nerve, and then applied GS to the lesion. Then we observed regeneration of axon and non-neuron.
Results: 1. NF-200 protein immunostaining for the visualization of axons showed more distal elongation of sciatic nerve axons in GS-treated group than saline-treated control 3 and 7 days after crush injury. 2. GAP-43 protein was increased in the injured sciatic nerve and further increased by GS treatment. Enhanced GAP-43 protein signals were also observed in DRG prepared from the rats given nerve injury and GS treatment. 3. GS treatment in vivo induced enhanced neurite outgrowth in preconditioned DRG sensory neurons. In vitro treatment of GS on sensory neurons from intact DRG also caused increased neurite outgrowth. 4. Phospho-Erk1/2 protein levels were higher in the injured nerve treated with GS than saline. Phospho-Erk1/2 protein signals were mostly found in the axons in the injured nerve. 5. NGF and Cdc2 protein levels showed slight increases in the injured nerves of GS-treated group compared to saline-treated group. 6. The number of Schwann cell population was significantly increased by GS treatment in the injured sciatic nerve. GS treatment with cultured Schwann cells increased proliferation and Cdc2 protein signals. 7. GS pretreatment into the injured spinal cord generated increased astrocyte proliferation and oligodendrocytes in culture. In vitro treatment of GS resulted in more differentiated pericytoplasmic processes compared with saline treatment. 8. More arborization around the injury cavity and the occurrence at the caudal region of CST axons were observed in GS-treated group than in saline-treated group.
Conclusion: GS extract may have the growth-promoting activity on regenerating axons in both peripheral and central nervous systems.
Axonal and dendritic extension by protopanaxadiol-type saponins from ginseng drugs in SK-N-SH cells.
Tohda C1, Matsumoto N, Zou K, Meselhy MR, Komatsu K. (2002)
Extension of axons and dendrites in neurons may compensate for and repair damaged neuronal networks in the dementia brain. To find out drugs capable of regenerating the neuronal network, we focused on several herbal drugs belonging to the genus Panax, kinds of Ginseng, and investigated neurite outgrowth activity of their extracts and compounds. We found that the methanol extracts of Ginseng (root of P. ginseng), Notoginseng (root of P. notoginseng) and Ye-Sanchi in Chinese (rhizome of a relative to P. vietnamensis) increased neurite outgrowth in SK-N-SH cells. The protopanaxadiol-type saponins, ginsenosides Rb(1) and Rb(3), and notoginsenosides R(4) and Fa isolated from Ye-Sanchi extract extended neurites, while protopanaxatriol-, ocotillol- and oleanane-type saponins had no effect on the neurite outgrowth. The percentage of cells with multipolar neurites and number of varicosities were intensely high in cells treated with the methanol extract of Ye-Sanchi as well as ginsenosides Rb(1) and Rb(3), and notoginsenosides R(4) and Fa. Both phosphorylated NF-H-expressing neurites and MAP2-expressing ones were extended by treatment with those saponins and the extract. Especially, longer neurites were mainly positive for phosphorylated NF-H. These results suggest that protopanaxadiol-type saponins enhance axonal and dendritic formation activity.
Pa nax notoginseng saponins improve recovery after spinal cord transection by upregulating neurotrophic factors
Bo Wang, Yu Li,* Xuan-peng Li, and Yang Li (2015)
Saponins extracted from Panax notoginseng are neuroprotective, but the mechanisms underlying this effect remain unclear. In the present study, we established a rat model of thoracic (T10) spinal cord transection, and injected Panax notoginseng saponins (100 mg/kg) or saline 30 minutes after injury. Locomotor functions were assessed using the Basso, Beattie, and Bresnahan (BBB) scale from 1 to 30 days after injury, and immunohistochemistry was carried out in the ventral horn of the spinal cord at 1 and 7 days to determine expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). Our results show that at 7–30 days post injury, the BBB score was higher in rats treated with Panax notoginseng saponins than in those that received saline. Furthermore, at 7 days, more NGF- and BDNF-immunoreactive neurons were observed in the ventral horn of the spinal cord of rats that had received Panax notoginseng saponins than in those that received saline. These results indicate that Panax notoginseng saponins caused an upregulation of NGF and BDNF in rats with spinal cord transection, and improved hindlimb motor function.
You might say the last one is cheap, that things that upregulate NGF and BDNF are a dime a dozon. Yes but ginseng also makes the list for GDNF, making it one of the very few natural compounds that stimulates all three common neurotrophins. Additionally, there is a lot of overlap between neurotrophin boosters and axon regeneration. GDNF boosters include catalpol, harpagoside, gastrodin, bilobalide, ginsenosides, naringin, royal jelly, and surprisingly ibogaine. Baicalin and conophylline promote β-cell differentiation through NeuroD1. Possibly helpful.
More studies:
The effects of Ginkgo biloba extract (GBe) on axonal transport microvasculature and morphology of sciatic nerve in streptozotocin-induced diabetic rats
Jinman Kim, Kazuhito Yokoyama, and Shunichi Araki (2000)
To evaluate the protective effects ofGinkgo biloba extract (GBe) which has antioxidant activity against peripheral neuropathy due to diabetes mellitus, slow axonal transport and morphology of sciatic nerve including endoneurial microvessels were examined in 12 rats with diabetes mellitus induced by streptozotocin (STZ, 60mg/kg, b.w., i.p.). Six of the diabetic rats were treated with 0.1 % of GBe for 6 weeks from one week after the STZ injection. Serum glucose and lipid peroxide levels in GBe-treated diabetic rats were significantly lower than those in untreated diabetic rats (p<0.01, respectively), though the serum glucose level was higher than that in the control rats. L-[35S] methionine pulse radiolabeling with subsequent gel fluorography demonstrated that mean velocities (Vmean) of actin and β-tubulin, i.e. slow component b (SCb) transport in untreated diabetic rats were significantly lower than those in control rats (p<0.05, respectively); mean diameter of axons in the former rats was significantly smaller than that in the latter (p<0.01). Vmean of actin transport in GBe-treated diabetic rats was significantly faster than that in untreated diabetic rats (p<0.05). Vmean of slow axonal transport was significantly correlated with mean diameter of axons in the three groups of rats combined (p<0.01). On electron microscopy, severe altered endoneurial microvessels decreasing in luminal area together with endothelial cell degeneration or hypertrophy, pericyte debris and basement membrane thickening were observed in untreated diabetic rats; on the other hand these findings were less prominent in the diabetic rats treated with GBe. It is suggested that GBe treatment may protect disturbed slow axonal transport and pathological alterations of peripheral nerve with abnormal endoneurial microvasculature from diabetes mellitus by antioxidant activity.
Semaphorins in axon regeneration: developmental guidance molecules gone wrong?
R. Jeroen Pasterkamp, and Joost Verhaagen (2006)
The biological role of vertebrate MICALs is currently unknown. Expression analysis and yeast interaction experiments hint at a role downstream of Sema3s and their receptors, plexinAs (Terman et al. 2002; Pasterkamp et al. 2005). This idea gains further support from the observation that certain green tea polyphenols with the ability to block flavoprotein monooxygenases, (−)-epigallocatechin gallate (EGCG) and (−)-epicatechin (EC) (Abe et al. 2000a,b), abrogate Sema3A- and Sema3F-mediated repulsion of rat sensory axons in vitro (Terman et al. 2002; Pasterkamp et al. 2005).
The Flavonoid Isoquercitrin Promotes Neurite Elongation by Reducing RhoA Activity
Gemma Palazzolo, Peter Horvath, Marcy Zenobi-Wong, and *Alexander G. Obukhov, Editor (2012)
Neurite formation and synaptic patterning are fundamental to the development of a functional nervous system. Flavonoids are natural molecules known for having beneficial effects on brain health through diverse molecular pathways. Cytoskeletal changes occurring during neuritogenesis and synapse formation often involve Rho GTPases. Here we hypothesized that the flavonoid isoquercitrin promotes neuronal differentiation through Rho signalling.
Enhancement of mouse sciatic nerve regeneration by the long chain fatty alcohol, N-Hexacosanol.
Azzouz M1, Kenel PF, Warter J-M, Poindron P, Borg J. (1996)
The purpose of the present study was to determine the effects of n-hexacosanol (hexa) on nerve regeneration. Hexa, a long chain fatty alcohol has been shown to possess neurotrophic properties on cultured neurons and to attenuate the degeneration of cholinergic neurons after injury. The effects of daily intraperitoneal injections of hexa (1 mg/kg) on regeneration of nerve fibers were studied in mice following a sciatic nerve crush. Measurement of axonal regeneration using the pinch test 7 days postlesion showed a 40% increase of the regeneration rate of sensory fibers in hexa-treated mice compared to controls (1.67 +/- 0.15 mm/day and 1.09 +/- 0.03 mm/day, respectively). The recovery of neuromuscular function was significantly improved, as shown by quantitative electromyography and and sensorimotor tests. Clinical signs of recovery evaluation with toe spreading reflex appeared earlier in hexa group than in control animals. Electrophysiological recordings were performed each 3 days during 34 days following nerve injury. Higher values of the compound muscle action potential (CMAP) were obtained in hexa-treated animals that correspond to an improved regeneration. Moreover, hexa induced a significantly faster regeneration rate (hexa: 2.87 +/- 0.15 mV/day; control: 2.00 +/- 0.06 mV/day), as measured by the slope of CMAP increase (44% enhancement). A morphometric analysis performed 7 days following crush showed an increased number of regenerating fibers, as well as increased diameter and thickness of the myelin in hexa-treated mice. Thus, hexa increased the regeneration of both sensory and motor axons in lesioned nerve, leading to an improved functional recovery.
Valproic acid enhances axonal regeneration and recovery of motor function after sciatic nerve axotomy in adult rats
Shu-Sen Cuia, c, Christine P. Yangb, Rudy C. Bowena, Ou Baia, Xin-Min Lia, Wen Jianga, Xia Zhanga (2003)
It has recently been demonstrated that valproic acid (VPA) robustly promotes neurite outgrowth, activates the extracellular signal regulated kinase pathway, and increases growth cone-associated protein 43 and bcl-2 levels in cultured human neuroblastoma SH-SY5Y cells. We hypothesized that VPA could also enhance peripheral nerve regeneration in adult animals. To test this hypothesis, we examined the effects of VPA (300 mg/kg daily for 16 weeks) on sciatic axonal regeneration following single or conditional axotomies in rats. The results showed that in VPA-treated rats there was a significant increase in the total numbers of regenerated myelinated nerve fibers and reinnervated muscle fibers in comparison with those rats not treated with VPA. As measured by sciatic function index and toe spread index, the motor function of the reinnervated hind limbs of rats receiving single axotomy without VPA treatment significantly improved at week 8 and reached plateau levels at about week 11, whereas the motor function of the reinnervated hind limbs of rats receiving single axotomy plus VPA and rats receiving conditional axotomy with or without VPA treatment significantly improved at week 4 and reached plateau levels at about week 8; there was no significant difference of the motor function among the three later groups. The results demonstrated that VPA is able to enhance sciatic nerve regeneration and recovery of motor function in adult rats, suggesting the potential clinical application of VPA for the treatment of peripheral nerve injury in humans.
Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain
Y T Wei, W M Tian, X Yu, F Z Cui, S P Hou, Q Y Xu, and In-Seop Lee (2003)
A biocompatible hydrogel of hyaluronic acid with the neurite-promoting peptide sequence of IKVAV was synthesized. The characterization of the hydrogel shows an open porous structure and a large surface area available for cell interaction. Its ability to promote tissue repair and axonal regeneration in the lesioned rat cerebrum is also evaluated. After implantation, the polymer hydrogel repaired the tissue defect and formed a permissive interface with the host tissue. Axonal growth occurred within the microstructure of the network. Within 6 weeks the polymer implant was invaded by host-derived tissue, glial cells, blood vessels and axons. Such a hydrogel matrix showed the properties of neuron conduction. It has the potential to repair tissue defects in the central nervous system by promoting the formation of a tissue matrix and axonal growth by replacing the lost tissue. [this one is interesting, heard they were looking at spider (or slug?) silk as a natural implant!]
Retinoic acid signaling in axonal regeneration
Radhika Puttagunta, and Simone Di Giovanni (2011)
Following an acute central nervous system (CNS) injury, axonal regeneration and functional recovery are extremely limited. This is due to an extrinsic inhibitory growth environment and the lack of intrinsic growth competence. Retinoic acid (RA) signaling, essential in developmental dorsoventral patterning and specification of spinal motor neurons, has been shown through its receptor, the transcription factor RA receptor β2 (RARβ2), to induce axonal regeneration following spinal cord injury (SCI). Recently, it has been shown that in dorsal root ganglion neurons (DRGs), cAMP levels were greatly increased by lentiviral RARβ2 expression and contributed to neurite outgrowth. Moreover, RARβagonists, in cerebellar granule neurons (CGN) and in the brain in vivo, induced phosphoinositide 3-kinase dependent phosphorylation of AKT that was involved in RARβ-dependent neurite outgrowth. More recently, RA-RARβpathways were shown to directly transcriptionally repress a member of the inhibitory Nogo receptor (NgR) complex, Lingo-1, under an axonal growth inhibitory environment in vitro as well as following spinal injury in vivo. This perspective focuses on these newly discovered molecular mechanisms and future directions in the field.
Even more:
Enhancement of Amygdaloid Neuronal Dendritic Arborization by Fresh Leaf Juice of Centella asiatica
http://www.hindawi.c...009/247831/abs/
Centella asiatica Leaf Extract Treatment During the Growth Spurt Period Enhances Hippocampal CA3 Neuronal Dendritic Arborization
http://www.hindawi.c...006/627102/abs/
Altered dendritic arborization of amygdala neurons in young adult rats orally intubated with Clitorea ternatea aqueous root extract
http://onlinelibrary...r.1657/abstract
Enhancement of basolateral amygdaloid neuronal dendritic arborization following Bacopa monniera extract treatment in adult rats
http://www.scielo.br...ipt=sci_arttext
Enhanced dendritic arborization of amygdala neurons during growth spurt periods in rats orally intubated with Bacopa monniera extract
http://link.springer...2565-011-0104-z
Curcumin protects axons from degeneration in the setting of local neuroinflammation
http://www.ncbi.nlm....pubmed/24382451
Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury.
http://www.ncbi.nlm....pubmed/23669643
Low-Dose Curcumin Stimulates Proliferation, Migration and Phagocytic Activity of Olfactory Glial Cells
http://journals.plos...al.pone.0111787
Berberine Promotes Axonal Regeneration in Injured Nerves of the Peripheral Nervous System
http://www.ncbi.nlm....les/PMC3308712/
Enhanced survival and regeneration of axotomized retinal ganglion cells by a mixture of herbal extracts.
Cheung ZH1, So KF, Lu Q, Yip HK, Wu W, Shan JJ, Pang PK, Chen CF. (2002)
The aim of this study is to investigate the effects of Panax quinquefolius L. extract (PQE), Ginkgo biloba extract (GBE), and Hypericum perforatum extract (HPE), in combination or alone, on the survival and regeneration of axotomized retinal ganglion cells (RGCs) in an optic nerve transection model in adult hamsters. Unilateral transection of the optic nerve was performed to evaluate the effects of herbal extracts on the survival of axotomized RGCs. Effects of the herbal extracts on axonal regeneration of axotomized RGCs, on the other hand, were studied by attaching a peripheral nerve graft onto the transected ocular stump to induce regeneration. Operated animals received daily oral administration of vehicle or herbal extracts (PQE, GBE, and HPE), alone or in combination, for 7 and 21 days, respectively, in the survival and regeneration experiments. Surviving and regenerating RGCs were retrogradely labeled with Fluoro-Gold. The eyes were then enucleated and the retinas were flat-mounted for the counting of the labeled RGCs. Treatment with PQE, GBE and HPE alone failed to offer neuroprotection to injured RGCs. However, treatment with Menta-FX, a mixture of PQE, GBE, and HPE, significantly augmented RGC survival 7 days postaxotomy. Treatment with Menta-FX also induced a significant (87%) increase in the number of regenerating RGCs 21 days after optic nerve transection. This study demonstrates that herbs can act as a potential neuroprotective agent for damaged RGCs. It also suggests that the therapeutic value of herbal remedies can be maximized by the use of mixtures of appropriate herbs.
Growth-promoting activity of Hominis Placenta extract on regenerating sciatic nerve
http://www.ncbi.nlm....pubmed/16364210
Hedysa ri Extra ct Improves Regeneration after Peripheral Nerve Injury by Enhancing the Amplification Effect
http://journals.plos...al.pone.0067921
--------
Endocannabinoid-Goα signalling inhibits axon regeneration in Caenorhabditis elegans by antagonizing Gqα-PKC-JNK signalling.
Pastuhov SI1, Fujiki K, Nix P, Kanao S, Bastiani M, Matsumoto K, Hisamoto N. (2012)
The ability of neurons to regenerate their axons after injury is determined by a balance between cellular pathways that promote and those that inhibit regeneration. In Caenorhabditis elegans, axon regeneration is positively regulated by the c-Jun N-terminal kinase mitogen activated protein kinase pathway, which is activated by growth factor-receptor tyrosine kinase signalling. Here we show that fatty acid amide hydrolase-1, an enzyme involved in the degradation of the endocannabinoid anandamide (arachidonoyl ethanolamide), regulates the axon regeneration response of γ-aminobutyric acid neurons after laser axotomy. Exogenous arachidonoyl ethanolamide inhibits axon regeneration via the Goα subunit GOA-1, which antagonizes the Gqα subunit EGL-30. We further demonstrate that protein kinase C functions downstream of Gqα and activates the MLK-1-MEK-1-KGB-1 c-Jun N-terminal kinase pathway by phosphorylating MLK-1. Our results show that arachidonoyl ethanolamide induction of a G protein signal transduction pathway has a role in the inhibition of post-development axon regeneration.
ß-adrenoceptor blockers increase cardiac sympathetic innervation by inhibiting autoreceptor suppression of axon growth
http://www.ncbi.nlm....pubmed/20844139
Retina-derived growth-promoting extract supports axonal regeneration in vivo
http://www.sciencedi...006899386908498
Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/mTOR Pathway
Kevin Kyungsuk Park, Kai Liu, Yang Hu, Patrice D. Smith (2008)
The failure of axons to regenerate is a major obstacle for functional recovery after central nervous system (CNS) injury. Removing extracellular inhibitory molecules results in limited axon regeneration in vivo. To test for the role of intrinsic impediments to axon regrowth, we analyzed cell growth control genes using a virus-assisted in vivo conditional knockout approach. Deletion of PTEN (phosphatase and tensin homolog), a negative regulator of the mammalian target of rapamycin (mTOR) pathway, in adult retinal ganglion cells (RGCs) promotes robust axon regeneration after optic nerve injury. In wild-type adult mice, the mTOR activity was suppressed and new protein synthesis was impaired in axotomized RGCs, which may contribute to the regeneration failure. Reactivating this pathway by conditional knockout of tuberous sclerosis complex 1, another negative regulator of the mTOR pathway, also leads to axon regeneration. Thus, our results suggest the manipulation of intrinsic growth control pathways as a therapeutic approach to promote axon regeneration after CNS injury.
Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein
http://www.nature.co...s/403439a0.html
A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration
http://www.sciencedi...896627394900426Glial inhibition of CNS axon regeneration
Glenn Yiu & Zhigang He (2006)
Damage to the adult CNS often leads to persistent deficits due to the inability of mature axons to regenerate after injury. Mounting evidence suggests that the glial environment of the adult CNS, which includes inhibitory molecules in CNS myelin as well as proteoglycans associated with astroglial scarring, might present a major hurdle for successful axon regeneration. Here, we evaluate the molecular basis of these inhibitory influences and their contributions to the limitation of long-distance axon repair and other types of structural plasticity. Greater insight into glial inhibition is crucial for developing therapies to promote functional recovery after neural injury.
Axon Regeneration in the Peripheral and Central Nervous Systems
http://www.ncbi.nlm....846285/#S2title
Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors
http://www.nature.co...s/343269a0.html
Axonal Regeneration into Acellular Nerve Grafts Is Enhanced by Degradation of Chondroitin Sulfate Proteoglycan
http://www.jneurosci...6/6206.full.pdf
Genes Associated with Adult Axon Regeneration Promoted by Olfactory Ensheathing Cells: A New Role for Matrix Metalloproteinase 2
http://www.jneurosci...26/20/5347.full