Castiel, the Blasco study you post on the mice born with hyperlong telomeres is very interesting pertinent to this discussion.
https://www.nature.c...467-019-12664-x
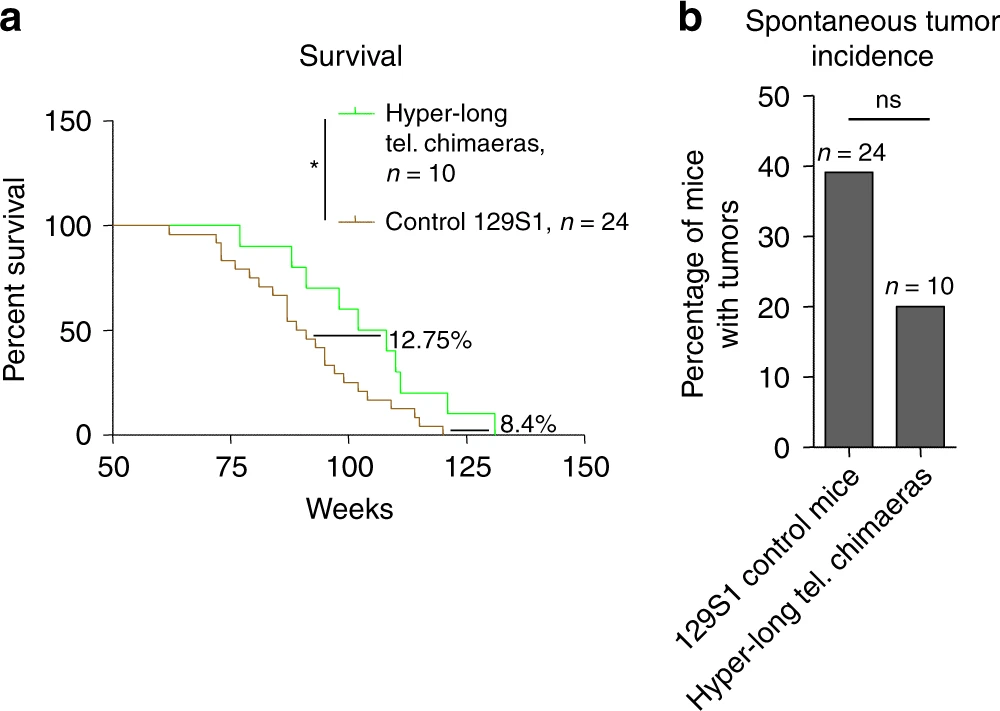
Hyper-long telomere mice are lean and show low cholesterol and LDL levels, as well as improved glucose and insulin tolerance. Hyper-long telomere mice also have less incidence of cancer and an increased longevity. These findings demonstrate that longer telomeres than normal in a given species are not deleterious but instead, show beneficial effects.
These mice were born from embryos that had intentionally been cultured for longer than would be the case in the murine womb, so benefited from additional telomere lengthening in embryonic cells (before differentiation). Their telomeres still shortened with age but started from a longer length.
I expect that this would also lead to the same phenomenon we are discussing - a longer time between stem cell replacements for a given somatic line. It would be interesting to see if these special mice had an epigenetic age that is older compared to control mice starting with normal length telomeres.
There is a human situation that is roughly analogous - the use of sartans. These were found to increase epigenetic age (I speculate through upregulated telomerase - see my thread) despite their health benefits.
https://www.ncbi.nlm...les/PMC6286862/
Accelerated DNA methylation age and the use of antihypertensive medication among older adults
The discrepancy of DNA methylation age (DNAmAge) with chronological age (termed as age acceleration, AA) has been identified to be associated with many aging-related health outcomes including hypertension. Since taking antihypertensive medication (AHM) could prevent aging-related diseases caused by hypertension, we hypothesized that using AHM could also reduce the AA....After the fully adjusting for potential covariates including hypertension, any AHM use showed a cross-sectional significant association with higher AA at each visit, as well as a longitudinal association with increased ΔAA between visits. Particularly, relative to participants who never took any AHM, individuals with continuous AHM use had a higher ΔAA of 0.6 year/chronological year. This finding underlines that DNAmAge and AA may not be able to capture the preventive effects of AHMs that reduce cardiovascular risks and mortality.